
Review Article
Austin Food Sci. 2020; 5(2): 1039.
Yeast-Derived Mannan Supplementation has Positive Effects on Immunity of Calves
Vaclav V¹* and De Oliveira Carlos AF²
¹Department of Pathology, University of Louisville, USA
²Department of Research and Development, Biorigin Company, Brazil
*Corresponding author: Vaclav Vetvicka, Department of Pathology, University of Louisville, USA
Received: May 25, 2020; Accepted: June 18, 2020; Published: June 25, 2020
Abstract
Farming faces numerous challenges: 1) emerging new diseases 2) current efforts to ban growth-promoting antibiotics 3) improve conditions and overall health of the farmed animals. This situation opens new opportunities for natural, highly effective and cost affordable immunomodulators. We evaluated the immunostimulative effects of a novel, yeast-derived mannan supplementation on health status of calves. Our results showed that 30-day supplementation resulted in significant improvements and offered two significant benefits: natural protection and natural growth stimulation.
Keywords: Immune; Mannan; Cattle; Cortisol; Phagocytosis; IL-2
Introduction
The immune system in general and innate immune system in particular, is fairly well conserved among all vertebrates. Neonatal calves’ intestinal tract develops immune mechanisms via passive and innate components of immunity. The health status of young calves is one of the most important factors contributing to their growth. A major issue is diarrhea, most often caused by Escherichia coli infection. Early stimulation of innate immunity is an important method to enable calves to thrive after maternal antibodies have waned and before their specific immunity is fully developed.
One possibility is the use of natural immunostimulants such as glucan or mannan.Glucan’s role as an immunomodulator has been well documented for over 50 years. β-Glucans show notable physiological effects; this is their most important quality and the reason why so much attention has been focused on them. β1,3- glucans, either particulate or soluble, exhibit immunostimulating properties, including antibacterial and anti-tumor activities [1,2]. Despite the fact that glucan has been intensively used in farm animals; the information on its effects in calves is sporadic.An interesting study showed that glucan addition to the vaccine-increased immune response [3]. Another study found improvements in milk quality and changes in cytokine expression after glucan administration [4]. A glucan-ascorbic acid combination modulated immune functions, particularly lung cell populations [5].
In the yeast cell wall, glucomannans are present in complex molecules usually linked to a protein moiety. Depending on isolation and size, the biological and immunological functions can vary [6]. One of the most studied components is Bio-Mos, a mannan oligosaccharide [7]. Glucomannan consists of glucose and mannose units joined by glycosidic linkages (via different positions and in different ratios). Similar to isolated glucans, glucomannan showed a broad range of biological activities including radioprotection, antimutagenic properties, anti-oxidative and immunostimulatory effects [8,9]. Readers seeking more information of immunological activities of various polysaccharides should seek an excellent review [10].
In this study, we evaluated the effects of a new generation of yeast wall containing soluble mannan and partial exposure of the beta-glucan layer into commercial feed of calves. We measured Body Weight (BW), changes in phagocytic activity, levels of cortisol after LPS challenge and effects of E. coli infection.
Material and Methods
Animals
At the onset of our study, all animals were evaluated for signs of any disease and all were considered healthy based on lacking any relevant abnormalities. Animal care and use was approved by the IACUC committee. Thirty calves (20 days old; 35±1, 5 kg of BW) were used for a 30-day experimental period. The animals were kept in individual pens. Weekly body weight was recorded and treatment dosages were adjusted accordingly.
The animals were chosen randomly forthe experimental treatments: Control, without yeast wall; or Mannan, with addition of 100 mg/kg BW yeast wall (Hypergen; BioriginCompany, Brazil) by gavage (Figure 1). On day 30 of experimentation, five animals in each group received LPS; fivemore animals in each group were challenged orally with E coli (see below). Composition of the feed is shown in Table 1. All animals were grown in conventional conditions.
Ingredient
% of diet
Corn silage
33.3
Alfaalfa silage
15.7
Alfaalfa hay
8
Corn, ground
15.5
Soybean meal
5.8
Corn hominy
5.2
Soybean hulls
4.9
Dried corn distillers grain
3.7
Canola meal
1.6
Soybean meal
3.7
Calcium carbonate
0.69
Molasses
0.58
Sodium bicarbonate
0.56
Sodium chloride
0.41
Mineral and vitamin mix
0.32
Monocalcium phosphate
0.032
Magnesium oxide
0.032
Monocalcium phosphate
0.069
Calcium sulfate
0.011
Table 1: Composition of diet.
Figure 1: Scheme of the experimental design.
Materials
Lipopolysaccharides (LPS from Salmonella enteriditis), Wright stain, and sodium citrate were obtained from Sigma (St. Louis, MO, USA).
Mannose
Biorigin R&D’s refined yeast-based mannan preparation was used in this study.New generation of yeast wall containing soluble mannan and partial exposure of the beta-glucan layer(Hypergen product).
Bacteria
Hemolytic E. coli strain GIS 26 was cultured during 18 hr. in Tryptone Soya Broth (Gibco). Bacteria were collected by centrifugation at 2800 mg for 45 min at 4oC. Bacteria were re-suspended in PBS and used at 109/ml [11]. Calves were orally inoculated with bacteria as described previously [12].
Phagocytosis
Phagocytic activity was evaluated as described earlier [13] using synthetic polymeric microspheres based on 2-hydroxyethyl methacrylate. Briefly: 0.1 ml of peripheral blood (with sodium citrate 1:9) was incubated in vitro with 0.05 ml of particles (diluted at 5x108/ml). The test tubes were incubated at 37oC for 60 min., with intermittent shaking. Smears were stained with Wright stain. The cells with three or more particles were considered positive. All experiments were performed in triplicate. At least 300 cells in 60 high power fields were examined in each experiment.
IL-2
Levels of IL-2 were evaluated in serum (1 ml of blood was collected) at the end of experimentation using a commercial bovine IL-2 ELISA kit as recommended by the manufacturer (Thermo Fisher Scientific, USA).
Evaluation of cortisol
Levels of cortisol were evaluated in serum (1 ml of blood was collected) at 1, 2 and 3 hrs. After injection of LPS from 30 days after beginning of feeding with mannan. The level of cortisol was measured using a commercial ELISA kit (Abnova, Walnut, MA, USA).
Body Weight
Calves were fed with the commercial diet formulated according to the NRC (2000; Table 1) with or without sample for 30 days. Weights were determined weekly to calculate Average Daily Gain (ADG) and Average Daily Feed Intake (ADFI).
Collection of samples
Feces were collected using plastic bags attached around the anus. Feces used for subsequent analysis of Short-Chain Fatty Acids (SCFA) were stored at -20oC. Just before testing, feces were finely ground to pass through a 0.5 mm mesh and analyzed for SCFA by gas chromatography as described previously [14].
IgA
IgA in the serum was detected by ELISA as described before [15]. Excretion of F4+ ETEC in feces was measured by dot-blotting hemolytic colonies after inoculation of 10-fold dilutions of feces on blood agar plates using F4-specific monoclonal antibody IMM01 as shown in [12].
Statistics analysis
Data was subjected to SAS (Version 9.1.3, 2004, SAS Institute Inc., Cary, NC), verifying the normality of residuals and homogeneity of variances by PROC UNIVARIATE. Outliers were removed to achieve normality of residuals when necessary. Student t-test was used to statistically analyze the data.
Results
Body weights were recorded every week for the entire duration of the study. ADGs were calculated for each of the 7-day periods. From data shown in Figure 2, it is clear that the ADG was positively influenced by mannan supplementation. Average daily feed intake showed significant improvements after longer supplementation (Figure 3).
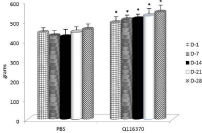
Figure 2: Average Daily Gain (ADG). Results are given as mean +/- SD.
*Represents significant differences at P≤ 0.05 level.
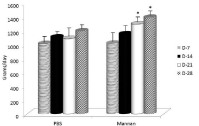
Figure 3: Average daily feed intake. Results are given as mean +/- SD.
*Represents significant differences at P≤ 0.05 level.
Mannans are known for their health effects. As phagocytosis is one of the first defense mechanisms, we decided to evaluate the effects of diet supplementation on phagocytic activity of blood neutrophils and monocytes. Using a test employing synthetic microspheres with minimal false positivity, we found that addition of our sample to the diet significantly stimulated phagocytic activity of both peripheral neutrophils and monocytes (Figure 4). Experiments testing the effects on IL-2 secretion in the blood showed strong stimulation (Figure 5).
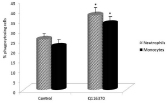
Figure 4: Effect of feeding on phagocytosis of peripheral blood neutrophils
and monocytes. Results are given as mean +/- SD. *Represents significant
differences at P≤ 0.05 level.
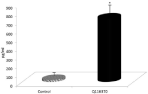
Figure 5: Effects of feeding on IL-2 formation. Results are given as mean
+/- SD. *Represents significant differences at P≤ 0.05 level.
The next experiments measured the effects of mannan supplementation of the body response after a lipopolysaccharide challenge. By evaluating the levels of the stress hormone cortisol, we found that mannan supplementation significantly abolished the effect of stress at 1 and 3 hr. intervals, the situation at 2 hr. was not statistically different (Figure 6).
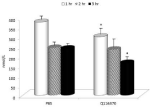
Figure 6: Effects of feeding on levels of cortisol. Results are given as mean
+/- SD. *Represents significant differences at P≤ 0.05 level.
The next part of our study focused on effects of E. coli infection. This infection induced a rapid increase in the F4-specific IgA antibodies, which was significantly higher in the supplemented group (Figure 7). When we evaluated the amount of F4+ E. coli in feces, we found significantly lower amounts in the supplemented group, except at day 4, where the trend was opposite (Figure 8). Furthermore, mean excretion in the control group was higher than in the supplemented group on every day post-infection, except on day 4, which might influence the results.
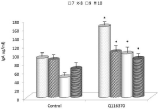
Figure 7: Day-dependent levels of F4-specific IgA antibodies. Results are
given as mean +/- SD. *Represents significant differences at P≤ 0.05 level.
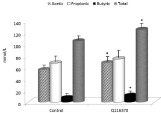
Figure 8: Effects of supplementation on production of acetic, propionic and
butyric short chain fatty acids. Results are given as mean +/- SD. *Represents
significant differences at P≤ 0.05 level.
In the last part of our study, we concentrated on the possible effects of mannan-feeding on the production of SCFA. Our results found that the supplementation increased the production of acetic, propionic and butyric short chain fatty acids (Figure 8).
Discussion
Mortality of young calves has been a major problem in dairy farming with up to 8% of dairy heifers dying prior to weaning an additional 1.9% dying post-weaning. Most common problemsare diarrhea, as young calves are highly susceptible to various infections resulting in the primary damage of the intestine. Management strategies to improve calf health, performance and immune functions are needed. The use of antibiotics would cure most of the problems, particularly neomycin has strong effects. However, most countries restricted or even prohibited the use of antibiotics in farmed animals, increasing the need to find an alternative.
Glucans, mannans or glucomannans represent a group of natural polysaccharides with demonstrated wide ranges of biological activities [16]. Most work on immune response stimulation in calves has focused on vaccines to stimulate specific immunity; lately the interest switched more towards ways to stimulate innate immunity. Yeast-derived mannans such as Bio-Mos have a long history of positive effects on farmed animals including chicken, fish and calves, reaching from improved antibody response to reduced mortality [17]. One of the primary functions of mannan oligosaccharides is to provide competitive binding to gram-negativebacteria and subsequently block them from colonizing the epithelium. Some studies show these compounds can alter the composition of the intestinal flora and improve intestinal health of calves [18]. Positive effects on macrophages and protection against viral infection were also reported [19,20]. Potential mechanisms of these actions might involve collections which production can be stimulated by mannan oligosaccharides [19]. Some studies suggest that mannan oligosaccharides can replace antibiotics completely [21].
The present study focused on evaluation of possible effects of insoluble mannan supplementation on various reactions in calves. We observed significantly improved ADG, which was particularly pronounced with length of food supplementation. This agrees with data from a previous study [22]. The exact mechanisms of these effects are not clear, as the energetic contribution of mannan is negligible. Stress reduction and/or direct effect on gastrointestinal tract conditions and microbiota might serve as a possible explanation.
With respect to farm animal immune systems, one must remember that these animals are naturally and constantly challenged by LPS during their entire life. During even simpler events such as changes in feeding or a subclinical disease, higher levels of LPS will be produced. We found significantly lower levels of the stress hormone cortisol, which is in agreement with our older study using a pig model [23].
Polysaccharides are known to be stimulators of immune reactions, particularly of the cellular branch. For evaluation of phagocytic activity, we used synthetic polymeric microspheres based on hydroxyethyl methacrylate, known for no false positive binding to the cells [13]. Our results showed significant stimulation of phagocytosis of peripheral blood cells, which is in agreement with previous studies on mice and pigs [23]. These effects are particularly important as neutrophils play a key role in initiating an innate immune response; they are the first cell type at the infection site. In addition to cellular immunity, we also evaluated the effects of food supplementation on IL-2 production. The results showed similar effects, suggesting that our material affects both branches of the immune system.
Post-weaning diarrhea represents a serious problem in farmed pigs and calves. Based on reports showing that yeast-derived polysaccharides can prevent this problem, we evaluated the possible effects of our material on E. coli challenge [24]. The specific anti-E. coli antibodies, which are the major preventive mechanisms of postweaning diarrhea, showed higher titers in supplemented animals. This concurs with previous studies in piglets and studies showing that polysaccharides may improve humoral immunity in response to vaccination or pathogen challenge [11,25,26]. Another positive influence might be the increase in health promoting bacteria in the intestine. The correct functional development of the gastrointestinal tract is of special importance during the weaning phase.
Calve’s intestinal microbiota change in response to dietary composition, due to the specific substrate preference of bacteria. Therefore, it can be assumed that addition of specific yeast-derived mannan to the diet allows manipulation of the composition of the intestinal microbiota. Particularly the butyrate is an important metabolite with a strong potential to affect gene expression and to improve cellular development in enterocytes [27]. Improved immune response and modulated intestinal microbiota by mannan oligosaccharides were also reported in carp and chicken [28,29].
Anaerobic gut bacteria in the cecum and large intestine produce Short Chain Fatty Acids (SCFA) as the end products of fermentation of mainly nondigestible carbohydrates passing the small intestine unaffected [30]. These SCFA exert multiple effects both on energy metabolism and on immunity [31,32]. SCFA influence the immune system through free fatty acid receptors 2 and 3. FFA2 is expressed primarily in leukocytes and colonic L-cells, partially in adipocytes whereas FFA3 is expressed mainly in adipocyte [33]. Various dietary fibers may interact directly with immunocompetent cells, like mucosal macrophages and dendritic cells, which exert pattern recognition receptors with carbohydrate-binding domains and decreases IL-12 and increases in IL-10 production [34]. On the other hand, there may be other mechanisms by which dietary fibers influence metabolism and immunity that may not be principally the same; they act as an SCFA source.
To conclude, the present findings indicated: 1) supplementation of food with yeast-derived mannan improves daily gains 2) improvesimmune function 3) reduces stress 4) alters SCFAs formation and intestinal microbiota. The mechanisms of these effects are probably a combination of direct effects on immune system and well known ability to directly affect gut microflora [35]. Our data supports the hypothesis that our yeast-derived mannan has strong and positive effect on calves and is able to significantly improve their biological and immunological conditions. Additionally, with current ban on antibiotics and societal fear of food chemicals, this product used as feed additive offers two significant benefits-natural protection and natural growth stimulation.
Acknowledgement
The authors would like to thank Biorigin for their donation of material and financial support. The funders had no role in the study design, data collection and analysis, or decision to publish. The authors have no competing interests to declare.
Author Disclosure Statement
V.V. has no conflict of interest. C.A.F.O. is employed by Biorigin. The study was financed by Biorigin (preparation, isolation and characterization of samples). The funders had no role in study resign, data collection and analysis, or decision to publish.
References
- Novak M, Vetvicka V. Beta-glucans history and the present immunomodulatory aspects and mechanisms of action J Immunotoxicol. 2008; 5: 47-57.
- Novak M, Vetvicka V. Glucans as biological response modifiers. EndocrMetab Immune Disord Drug Targets. 2009; 9: 67-75.
- Mansilla FC, Czepluch W, Malacari DA. Dose-dependent immunogenicity of a soluble Neospora caninum tachyzoite-extract vaccine formulated with a soy lecithin/beta-glucan adjuvant in cattle Vet Parasitol. 2013; 197: 13-21.
- Uchiyama H, Iwai A, Asada Y. A small scale study on the effects of oral administration of the beta-glucan produced by Aureobasidium pullulans on milk quality and cytokine expressions of Holstein cows, and on bacterial flora in the intestines of Japanese black calves BMC Res Notes. 2012; 5: 189.
- Eicher SD, Patterson JA, Rostagno MH. Glucan plus ascorbic acid in neonatal calves modulates immune functions with and without Salmonella enterica serovar Dublin Vet Immunol Immunopathol. 2011; 142: 258-64.
- Domer JE. Candida cell wall mannan a polysaccharide with diverse immunologic properties.Crit RevMicrobiol. 1989; 17: 33-51.
- Spring P, Wenk C, Connolly A, Kiers A. A review of 733 published trials on Bio-Mos, a mannan oligosaccharide, and Actigen, a second generation mannose rich fraction, on farm and companion animals J Appl Anim Nutr. 2015; 3: 8.
- Drabikova K, Perecko T, Nosal R, Katarína B, Silvester Ponist, Danica. Glucomannan reduces neutrophil free radical production in vitro and in rats with adjuvant arthritis Pharmacol Res. 2009; 59: 399-403.
- Hajkova V, Svobodova A, Krejcova D, Milan CIZ, Vladimr V, Antonin L. Soluble glucomannan isolated from Candida utilis primes blood phagocytes Carbohydr Res. 2009; 344: 2036-41.
- Ferreira SS, Passos CP, Madureira P, Vilanova M, Coimbra MA. Structurefunction relationships of immunostimulatory polysaccharides: A review Carbohydr Polym. 2015; 132: 378-96.
- Stuyven E, Cox E, Vancaeneghem S, Arnouts S, Deprez P, Goddeeris BM. Effect of beta-glucans on an ETEC infection in piglets Vet Immunol Immunopathol. 2009; 128: 60-6.
- Van den Broeck W, Cox E, Goddeeri BM. Receptor-dependent immune responses in pigs after oral immunization with F4 fimbriae Infect Immun. 2009; 67: 520-6.
- Vetvicka V, Holub M, Kovaru H, Siman P, Kovaru, F. Alpha-fetoprotein and phagocytosis in athymic nude mice Immunol Lett. 1988; 19: 95-8.
- Oltramari CE, Napoles GG, De Paula MR, et al. Performance and metabolism of calves fed starter feed containing sugarcane molasses or glucose syrup as a replacement for corn. Asian-Australas J Anim Sci. 2016; 29: 971-8.
- Van der Stede Y, Cox E, Goddeeris BM. Antigen dose modulates the immunoglobulin isotype responses of pigs against intramuscularly administered F4-fimbriae. Vet Immunol Immunopathol. 2002; 88: 209-16.
- Persson Waller K, Gronlund U, Johannisson A. Intramammary infusion of beta1,3-glucan for prevention and treatment of Staphylococcus aureus mastitis. J Vet Med B Infect Dis Vet Public Health. 2003; 50: 121-7.
- Torrecillas S, Makol A, Caballero MJ, Robaina L, Real F, Sweetman J. Immune stimulation and improved infection resistance in European sea bass fed mannan oligosaccharides. Fish Shellfish Immunol. 2007; 23: 969-81.
- McGuirk SM. Reducing dairy calf mortality. Proc Am Assoc Bov Pract. 2007; 40: 126-31.
- Franklin ST, Newman MC, Newman KE, Meek KI. Immune parameters of dry cows fed mannan oligosaccharide and subsequent transfer of immunity to calves. J Dairy Sci. 2005; 88: 766-75.
- Magalhaes VJ, Susca F, Lima FS, Branco AF, Yoon I, Santos JE. Effect of feeding yeast culture on performance, health, and immunocompetence of dairy calves J Dairy Sci. 2008; 91:1497-509.
- Heinrichs AJ, Jones CM, Heinrichs BS. Effects of mannan oligosaccharide or antibiotics in neonatal diets on health and growth of dairy calves J Dairy Sci. 2003; 86: 4064-9.
- Ghosh S, Mehla RK. Influence of dietary supplementation of prebiotics on the performance of crossbred calves Trop Anim Health Prod. 2012; 44: 617-22.
- Vetvicka V, Oliveira C. D-Glucans modulate immune status in pigs: potential importance for efficiency of commercial farming Ann Transl Med. 2014; 2: 16.
- Jensen KH, Darngaard BM, Andresen LO, Jorgensen E, Carstensen L. Prevention of post weaning diarrhoea by a Saccharomyces cerevisiaederived product based on whole yeast Anim Feed Sci Tech. 2013; 183: 29-39.
- Wang Z, Shao Y, Guo Y, Yuan J. Enhancement of peripheral blood CD8+ T cells and classical swine fever antibodies by dietary beta-1,3/1,6-glucan supplementation in weaned piglets Transbound Emerg Dis. 2008; 55: 369-76.
- White LA, Newman MC, Cromwell GL, Lindemann MD. Brewers dried yeast as a source of mannan oligosaccharides for weanling pigs J Anim Sci. 2002; 80: 2619-28.
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon FEMS Microbiol Lett. 2002; 217: 133-9.
- Momeni-Moghaddam P, Keyvanshokooh S, Ziaei-Nejad S, Parviz Salati A, Pasha-Zanoosi H. Effects of mannan oligosaccharide supplementation on growth, some immune responses and gut lactic acid bacteria of common carp fingerlings Vet Res Forum. 2005; 6: 239-44.
- Pourabedin M, Chen Q, Yang M, Zhao X. Mannan xylooligosaccharides modulate caecal microbiota and expression of inflammatory-related cytokines and reduce caecalSalmonella enteritidiscolonisation in young chickens FEMS Microbiol Ecol. 2016; 93.
- Den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism J Lipid Res. 2013; 54: 2325-40.
- Chan GC, Chan WK, Sze DM. The effects of beta-glucan on human immune and cancer cells J HematolOncol. 2009; 2: 25.
- Wismar R, Brix S, Frokiaer H, Laerke HN. Dietary fibers as immunoregulatory compounds in health and disease. Ann N Y Acad Sci. 2010; 1190: 70-85.
- Vangaveti V, Shashidhar V, Jarrod G, Baune BT, Kennedy RL. Free fatty acid receptors emerging targets for treatment of diabetes and its complications Ther Adv Endocrinol Metab. 2010; 1: 165-75.
- Warnberg J, Gomez-Martinez S, Romeo J, Diaz LE, Marcos A. Nutrition inflammation and cognitive function. Ann N Y Acad Sci. 2009; 1153: 164-75.
- Jahanian R, Ashnagar M. Effect of dietary supplementation of mannanoligosaccharides on performance, blood metabolites, ileal nutrient digestibility, and gut microflora in Escherichia coli-challenged laying hens. Poult Sci. 2015; 94: 2165-72.