
Research Article
Austin Food Sci. 2022; 7(1): 1050.
Efficacy of Edible Coating Based on the Rice Bran Oil and Acetylated Potato Starch Carrier Natamycin on the Qualitative Properties of Grape
Jeivan AO, Taghizadeh M* and Yavarmanesh M
Department of Food Science and Technology, Ferdowsi University of Mashhad (FUM), Mashhad, Iran
*Corresponding author: Taghizadeh M, Department of Food Science and Technology, Ferdowsi University of Mashhad (FUM), P.O. Box: 91775-1163, Mashhad, Iran
Received: February 22, 2022; Accepted: March 17, 2022; Published: March 24, 2022
Abstract
Use of agricultural products and their wastes have provided edible coatings and films, which have been more developed in recent years. This type of the food storage system, especially for fruits has well increased the product’s shelf life greatly and has reduced wastes. Emulsion gel consisting 6.57 gr of the acetylated potato starch and 2 gr of the rice bran oil produced at 7 rpm homogenization rate was selected as the best combination of edible coating. Edible coating consisting the rice bran oil and acetylated potato starch (control) increased acidity but reduced pH and TSS during storage. Addition of the high concentrations (10 and 20 mg L-1) of the natamycin caused the reduction of spoilage agent, waste and weight loss of clusters; also, it caused to more brightness of the clusters and reduced pH and acidity during storage; however, TSS was increased.
Keywords: Edible coating; Emulsion gel; Rice bran oil; Acetylated potato starch; Natamycin; Grape
Introduction
Fruits are suitable environments for microbial growth due to high content of sugars and other nutrients as well as high humidity [1]. “Grape” (it was scientifically named Vitis vinifera) has been considered as an important fruit among its producing countries. Its preservation is difficult due to its perishable nature. Post-harvest fungal contamination causes a lot waste of the grape. Also, the quality and marketability of product are reduced. Botrytis cinerea mold is the most important agent of grape post-harvest corruption all over the world [2,3]. Therefore, manufacturers seek alternative approaches to maintain quality of grape. In the past, to increasing the shelf life of this product was being mainly used sulfur dioxide [4-6]. Since the remains of this compound has been dangerous for human health [7,8] and has caused harms to fresh fruits and vegetables; it must is going to follow the alternative and effective ways [9]. “Edible coating” is a thin layer of materials (mostly biopolymers) which extend the shelf life of foods by protecting them against the transport of gases, water vapor, soluble solids and mechanical damages [10]. Various materials including hydrocolloids (polysaccharides and proteins), lipids (fatty acids, glycerides and waxes) have been used to produce the edible coatings [11]. Advantages of polysaccharide-based coatings can be referred to polarity, inhibition against oxygen and gases, to be cheap, high transparency and production of mechanically resistant films [12]. “Starch” is one of the most important polysaccharides that has made the good films. The starch-based films often are transparent or semi-transparent without odor, taste and color. Potato starch produces gels with high viscosity due to its high phosphorus content [13]. “Acetylated starch” reduces gelatinization temperature and increases transparency, viscosity and freezing stability of the gel [14- 17]. Previous studies indicated effectiveness of starch-based coatings on storage life and control decay of the red grape and strawberry fruits [18,19]. Adding lipids to hydrocolloid compounds has improved their moisture-barrier properties due to hydrophobic properties of lipids [20]. “Bran” is by-product of the rice processing [21]. Its oil increases shelf life of fruits by reducing weight loss, transpiration and respiration, which are major agents of their spoilage in refrigerator [22]. Studies were done about effect of this substance on the kiwi, cherry tomatoes and low fat sausage production and the positive results were obtained about reducing decline factors of fruits quality by rice bran oil [23-25].
To increase the effect of edible films and coatings, bioactive substances such as antimicrobial and antioxidant compounds have been used in their formulation, which have been said to be “Active films and coatings”. Use of bacteriocins as natural antimicrobial substances in foods isn’t going to cause any problem for human [26]. “Natamycin” is one of these compounds. It is safe for the consumer and more effective at low concentrations. Orgesterol is the major sterol into the cell membrane of molds and yeasts. The natamycin has a high tendency to irreversible binding the Orgesterol and cause to destruction of the spoilage agents molds. Minimum inhibitory concentration of natamycin against almost all food pathogenic fungi is less than 29 ppm [27]. So far, the good reports have been provided the antibacterial effect of natamycin on some chemical properties and preservation of products such as the black olives, hami-melons and fresh strawberries [28,29].
This study is divided into two parts. In the first phase, optimization was performed on the production of edible coating based on Rice Bran Oil (RBO) and Acetylated Potato Starch (APS) at different homogenization rates. In the second phase, the antimicrobial effect of natamycin with optimal coating obtained from the first stage and individually on the grape spoilage (mold) was evaluated. The experimental treatments were also studied chemically and sensually.
Materials and Methods
Materials
Grape (Asgari cultivar) was obtained from Kashmar (Khorasan Razavi) with characteristics such as much sweet taste and high storage capacity. The berries were uniform in size and color (light-green). Clusters were washed with brine and dried at ambient temperature for 2 h just prior to the experiments. Then, the clusters, which were free from any disease, insect, injury or machine damage, were selected for further study.
Acetylated potato starch- produced under the protection of Denmark- was purchased from Arsha Pouyeh Company (Tehran- Iran). Edible refined rice bran oil was prepared by Saman Oil Company (Khorasan Razavi-Mashhad) with purity of 99.99%. Natamycin (1 gr) and Botrytis cinerea mold (IBRC-M 30162) were purchased from Parseh Bio In Company (Khorasan Razavi-Mashhad) and the Iran-Tehran Genetic and Biological Reserves Center in the form of cultured plates, respectively. All other chemicals were obtained from commercial sources and were of analytic grade.
Optimizing the production of emulsion gel based on acetylated potato starch and rice bran oil
Response surface method and optimal custom design (due to the different levels of coating components) were used for optimization with 20 treatments and two repetitions under three independent variables including acetylated potato starch at three levels (3, 5 and 7 gr), rice bran oil at five Levels (0, 0.5, 1, 1.5 and 2 gr) and homogenization rate at three levels (7, 14.5 and 22 rpm). Weight loss of coated grapes (percentage), the stability (percentage) and aw of emulsion gels (percentage) parameters were considered as test responses to investigate the effect of different levels of treatments on them. The weight loss of 20 treatments belonging to optimization stage was also calculated as a percentage with three repetitions every two weeks.
Edible coating was prepared by continuous mixing a certain amount of acetylated potato starch in 100 ml of distilled water through gelatinization of starch into a hot water bath at 90°C for 5 min [30]. After cooling (for 30 min), the resulting gel was homogenized by addition a certain amount of rice bran oil (gr) and 2% Tween 80 as emulsifier under a certain rate of Ultraturex (rpm) for 5 min (T25 digital, Made in Germany) [31].
Measurement of the physical properties of the emulsion gel was produced:
Determination of stability: To measure stability of emulsion gels, a certain amount of every sample was poured into falcon and centrifuged for 30 min under 3600 rpm at ambient temperature (D72, Made in Germany). The separated phase was removed from container by reversing the falcon on filter paper. The stability was calculated as a percentage based on the ratio of secondary weight of the falcon containing emulsion gel to its initial weight [32].
Determination of aw: To determine aw of gels was used a water activity meter (HYGROLAB-3, Made in Swiss). Thus, a certain weight of every coating sample was poured into device (at temperature of 20°C).
After establishing equilibrium, the amount of water activity was calculated based on the ratio of equilibrium respective humidity to 100 [33].
The computation of spores
Microbial culture was grown on the Potato Dextrose Agar (PDA) medium through incubation at 25°C for 5 d [34]. At the beginning of this section, 100 ml of distilled water with 0.5 ml of Tween 80 were mixed and autoclaved. Then, 9 ml of distilled water along with the sterile Tween were poured into plate containing mold (Botrytis cinerea) that had already been cultured to remove the mold spores from plate surface by washing. Afterward, the mixture of spores were removed by a sterile pipette and were poured into a test tube; some distilled water and Tween were again added to achieve an acceptable dilution. To calculate the number of spores, 10 μl of solution containing spores were poured on a Homocytometer Lam [35].
a = (The average number of spores × 25 × 50 × 1000) (1)
Evaluation antimicrobial activity of the natamycin
At this stage, the Tween 80, broth medium and natamycin (at 5 levels of 1.25, 2.5, 5, 10 and 20 mg L-1) at rate of 50 μl and 3 levels of spores including 104, 106 and 108 at rate of 2000 μl of Each level were used. Their absorbance was read at a wavelength of 600 nm by ELISA software, before and after incubation.
MIC (the lowest concentration of antifungal agent that shows any growth for each level of spore) and MFC (the lowest concentration of antimicrobial activity that kills 99.9% of microorganisms) were estimated. The experiments were performed in three repetitions.
Evaluation of the effect of natamycin in combination with edible coating on fruit quality characteristics (preparation treatments)
All process of preparation of the emulsion gels was done similarly in both phases of the study (section 2.2); but, in the second stage, natamycin added to optimal coating at different levels. One sample as control (containing edible coating without the presence of natamycin and mold spores) and subsequent treatments include edible coating with presence of mold spores at 3 levels (2000 μl each level of spore). Also, two higher concentrations of MIC value (mg L-1) of natamycin were considered for each level of spore. This section was performed with two repetitions (14 samples).
In this way, first, clusters were infected with mold spores by spray method and drying at room temperature for 1 hr. Then, they were immersed into edible coating so that all berries were coated for 1 min. When the coatings on the clusters (for 2 h) were dried, samples were placed in disposable plastic containers without cap and refrigerated for 4 weeks at suitable temperature and humidity (4°C and 70%) (Figure 1).

Figure 1: Coating the clusters of grape were contaminated by mold spores
with edible coating containing acetylated potato starch, rice bran oil and
natamycin (14 treatments).
Qualitative parameters of samples were measured every two weeks. Treatments were provided as follows (Table 1).
Treatment
Coatings formulations
Sample 1
APS (6.57gr)+ RBO (2gr) + 20 mg L-1 + 108 spores
Sample 2
APS (6.57gr)+ RBO (2gr)+ 10 mg L-1 + 108 spores
Sample 3
APS (6.57gr)+ RBO (2gr)+ 10 mg L-1 + 106 spores
Sample 4
APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores
Sample 5
APS (6.57gr)+ RBO (2gr)+ 5 mg L-1 + 104 spores
Sample 6
APS (6.57gr)+ RBO (2gr)+ 2.5 mg L-1 + 104 spores
Control
APS (6.57gr)+ RBO (2gr)
Table 1: Clusters were coated by composition constant (optimal) amounts of acetylated potato starch, rice bran oil and different levels of the natamycin and mold Botrytis cinerea spores.
Evaluation of the decay and weight loss of grape clusters during storage:
Determination of decay: Fresh clusters were examined for mold growth every two weeks of storage. The fruit was removed by observing the extent of mold micelles, creating brown and gray spots on surface of grape. Fruit spoilage was calculated as a percentage the amount of spoiled berries to total available berries [36].
Determination of weight loss: Coated treatments were weighed by digital Scale with accuracy of 0.001 ± 1 gr (Ouauz GT 2100, Made in USA) before and after storage in refrigerator. Their weight loss was reported as a percentage through the ratio of the difference between the initial weight of the cluster and its initial weight [37].
Measuring the chemical parameters of the grape clusters:
Determination of total soluble solid: To determine the Total Soluble Solid (TSS) of the samples, Refractometer (PAL-3, Made in USA) was used at three repetitions [23].
Determination of titratable acidity: The Titratable Acidity (TA) was measured using 5 ml of grape juice filtered in 95 ml of distilled water, 0.1 N NaOH and Plenolphthalein reagent to an end-point of pH 8.1 at three repetition [38].
Determination of pH: Grape extracted juice pH value was measured by a pH meter at three repetition (CP-744, Made in England) [39].
Determination of fruit’s color: Fruit’s color was measured by Image software. The CIE Laboratory color scale was used to determine the parameters L* (Black to White), a* (Red to Green) and b* (Yellow to Blue). All measurements were performed at three repetitions [40].
Sensory evaluation: The coated grapes were judged by 7 panelists to evaluate the sensory characteristics and general acceptance (in the terms of odor, color, appearance, texture and overall acceptability) every two weeks. To study the mentioned parameters, the 5-point Hedonic ranking method was used. The scores 5 and 1 were considered for a very good and bad attribute, respectively.
Statistical analysis
Design-Expert software (10.0.3.0-x64-Softcozar.com.msi) in the form of optimal custom design with 20 treatments, 3 measured variables and 3 responses were used to evaluate the results and determined the optimal conditions for the production of edible coating based on acetylated potato starch and rice bran oil. On the other hand, SPSS version22 software was used for statistical analysis. Duncan’s Multiple Range Tests (DMRT) with maximum acceptable error of 5% was used to measure significant differences between samples, Analysis Of Variance (ANOVA) and comparison treatment means. Also, Excel2013 software was carried to plot changes different of the parameters and graphs.
Results and Discussion
Results of edible coating optimization
The results obtained from effect of emulsion gels were produced (20 samples of edible coating) on the design responses (weight loss, stability and aw) is inserted in Table 2.
Observations
Acetylated Potato Starch
(gr)Rice Bran Oil
(gr)Homogenize speed (rpm)
Weight loss %
Stability %
aw %
1
7
1
14.5
5.44
88.6
0.8
2
7
2
22
4.94
93.6
0.81
3
7
0
22
18.21
76.75
0.84
4
7
1
14.5
5.9
91.94
0.86
5
7
0
7
6.17
91.46
0.9
6
7
2
7
6.83
66.52
0.77
7
7
0
22
5.67
74.29
0.83
8
7
0.5
7
5.11
72.25
0.81
9
3
0
7
5.17
80.26
0.82
10
3
2
22
2.47
78.73
0.78
11
7
0
22
5.11
91.85
0.89
12
7
1
7
4.5
89.61
0.86
13
3
1.5
14.5
6.1
76.26
0.79
14
3
0.5
14.5
6.3
69.52
0.81
15
5
1
22
4.3
86.15
0.83
16
3
0.5
14.5
5.046
76.56
0.81
17
5
2
14.5
4.054
84.54
0.78
18
5
0
14.5
4.52
80.21
0.82
19
7
2
7
2.44
90.69
0.79
20
5
1
22
4.2
84.34
0.8
Table 2: The results of the preparation of emulsion gels.
The value of p = 0.0015 for the model related to the weight loss of grape samples in the coating optimization stage indicated its compliance with experimental data (Table 3).
Variables
Regression coefficients
Degree of freedom
Sum of squares
Mean Square
F
P
X1(APS)
-0.44
1
2.02
2.02
12.07
0.0052**
X2(RBO)
+9.906E -004
1
-006 8.203E
-006 8.203E
4.90E-05
0.9945ns
X2 X1
-0.6
1
2.17
2.17
12.96
0.0042**
X12
0.93
1
2.66
2.66
15.91
0.0021**
Model
-
4
6.29
1.57
9.39
0.0015**
Residual
-
11
1.84
0.17
-
-
Lack of fit
-
8
0.94
0.12
0.39
0.8695ns
Pure Error
-
3
0.9
0.3
-
-
Total
-
16
10.52
-
-
-
APS: Acetylated Potato Starch; RBO: Rice Bran Oil.
Note: R2 = 0.77, R2adj =0.69
Table 3: ANOVA results for the quadratic equation for weight loss.
Considering the value of R² = 0.77, it can be concluded that regression model abled to predict the relationship between the independent variables (acetylated potato starch, rice bran oil and Stirring speed) and the dependent variables (weight loss). The secondorder linear regression equation is represented by Equation 2:
Y1 = +10.03724 - 2.24489X1 - 0.30214X1X2 + 0.23290X1 ² (2)
The results of analysis variance for the linear equation of the emulsion gel stability are shown in Table 4.
Variables
Regression coefficients
Degree of freedom
Sum of squares
Mean Square
F
P
X1(APS)
6.93
1
552.65
552.65
135.18
X2(RBO)
0.76
1
5.48
5.48
1.34
0. 2697ns
X3(volume†)
0.15
1
0.2
0.2
0.049
0.8281ns
Model
-
3
640.45
213.48
52.22
<0.0001****
Residual
-
12
49.06
3.93
-
-
Lack of fit
-
9
38.82
4.07
1.26
0.4714ns
Pure Error
-
3
10.24
3.41
-
-
Total
-
16
689.51
-
-
-
APS: Acetylated Potato Starch; RBO: Rice Bran Oil.
Note: R2 = 0.92, R2adj =0.91
Table 4: ANOVA results for linear equation related to stability.
The value of ****p <0.0001 obtained for the model indicated its agreement with the experimental data which according to the value of R² = 0.92 abled to predict the relationship between the independent and dependent variables. The concentration of acetylated potato starch (X1) had great effect on the stability of emulsion gel (p <0.0001). The linear regression equation of this parameter is given in Equation 3:
Y2 = +65.77187 + 3.46706X1 (3)
Table 5 shows the analysis variance of the linear equation aw (dependent variable). The value of p < 0.0001 for the model determined its consistency with data. Also, R2 = 0.82 confirmed the predictive power of model in terms of the relationship between variables. The linear equation of the effect of independent variables on the amount of aw was written in the form of Equation 4:
Variables
Regression coefficients
Degree of freedom
Sum of squares
Mean Square
F
P
X1(APS)
0.026
1
8.950E-003
8.950E-003
30.41
<0.0001****
X2(RBO)
-0.032
1
0.012
0.012
39.96
<0.0001****
X3(volume)
+3.866E -003
1
1.883E-004
1.883E-004
0.64
0.4371ns
Model
-
3
0.019
6.354E-003
21.95
<0.0001****
Residual
-
14
4.120E-03
2.943E-003
-
-
Lack of fit
-
11
3.620E-003
3.291E-004
1.97
0.3142ns
Pure Error
-
3
5.000E-004
1.667E-004
-
-
Total
-
18
0.023
-
-
-
APS: Acetylated Potato Starch; RBO: Rice Bran Oil.
Note: R2 = 0.82, R2adj =0.78
Table 5: ANOVA results for linear equation related to aw.
Y3 = +0.77958 + 0.013048X1 - 0.031816X2 (4)
The effect of different levels of acetylated potato starch and rice bran oil on weight loss: As it can be seen, first, weight loss decreased and then increased with increasing the concentration of APS (Figure 2).
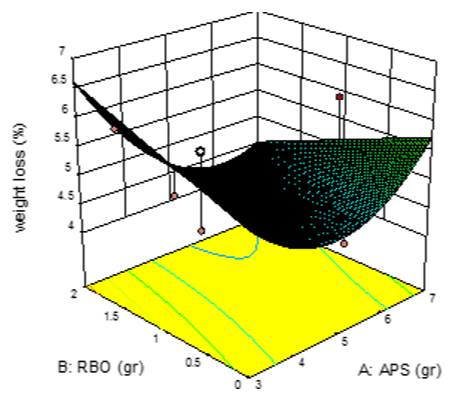
Figure 2: The effect of different levels of Acetylated Potato Starch and Rice
Bran Oil on the weight loss of the clusters.
This difference in result can be due to the inherent moisture loss of the fruit during storage, its weight was reduced. In the following, the environment slowed down the process of weight loss of grape by keeping fruit moisture; it even increased the weight of samples. Also, increasing the concentration of RBO had the significant effect on the weight loss so that the lowest percentage of weight loss was observed in the high concentrations both the independent variables. Therefore, rice bran oil has been able to prohibit from moisture loss of grape during storage by preventing the transmission of its.
In a study based on the weight loss of kiwi, fruit was coated by combination of the whey protein and rice bran oil and were kept at 8°C for 4 weeks. The results showed that the lowest weight loss was attributed to fruits containing the highest amount of Oil [23].
The above results were matched with a report based on the coating lemons and peppers by rice bran wax [41].
The effect of different levels of acetylated potato starch, rice bran oil and stirring speed on the stability of emulsion gel: According to Figure 3, the stability went through upward trend with increasing the concentration of APS.
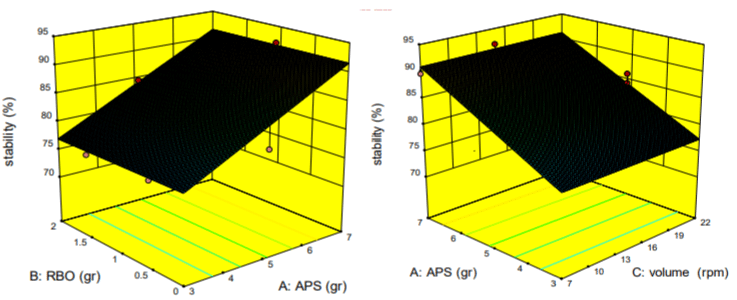
Figure 3: The effect of different levels of Acetylated Potato Starch, Rice Bran Oil and the Speed of stirring on the stability of the emulsion gel.
The highest stability was obtained at the highest level of acetylated potato starch. But none of the values of RBO and homogenization rate had any effect on the stability of emulsion gel. The reason for this could be the more and better strength of gel due to fill the maximum of empty spaces between two continuous and diffused phases. Also, uniform and more mixing the components of coating increased its stability that gelling agent (acetylated potato starch) was the effective parameter of this flow.
In a study, Researchers have achieved that hydrocolloids essentially are the hydrophilic molecules. Their presence decrease the rate of creaming and increase the stability of emulsion gel by increasing the continuous aqueous phase around the dispersed phase droplets and stabilizing them in a gel-like network [42]. Results obtained on the coated sausage with Inulin and rice bran oil showed that the stability of emulsion gel increased by using the high amounts of Inulin as a hydrocolloid [25].
The effect of different levels of acetylated potato starch, rice bran oil and stirring speed on the amount of emulsion gel aw: Figure 4 showed clearly the effect of different levels of acetylated potato starch, rice bran oil and stirring speed on water activity.
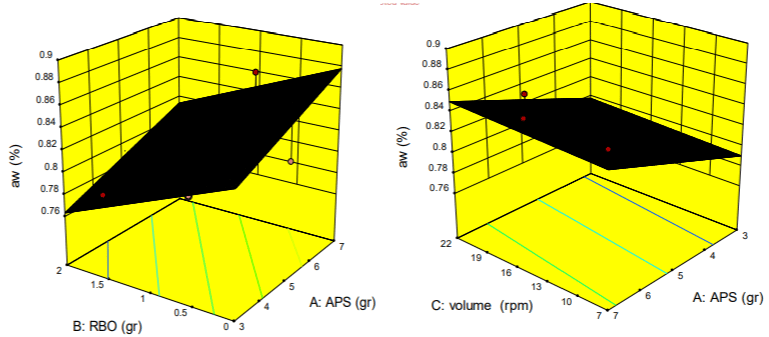
Figure 4: The effect of different levels of Acetylated Potato Starch, Rice Bran Oil and the Speed of stirring on the amount of aw emulsion gel.
Increasing the concentration of both acetylated potato starch and rice bran oil had the very significant effect (p < 0.0001) on the changes of water activity parameter. The effect was impressive to some extent that the highest and lowest aw were obtained at the minimum (0 gr) and maximum (3 gr) of RBO, respectively. But in the case of APS, results were significantly opposite obtained; thus, the water activity of emulsion gel increased with use of the high levels of starch. Also, the different levels of X3 had no effect on the water activity of gel.
Starch-based films in combination with a lipid nano-layer (such as sunflower oil) due to increase tensile strength, reduce water diffusion coefficients and water activity [43]. Since, these films are sensitive to moisture can reduce their water content by adding oils [44]. Considering the above items, it can be concluded that a desirable water content (the less than 0.8%) is going to create for the emulsion gel by increasing the level of consumption of rice bran oil as a plasticizer in the composition of edible coating.
Developed models related to the weight loss of samples, the stability and aw of emulsion gel predicted that using of 2 gr rice bran oil, 6.57 gr acetylated potato starch and 7 rpm homogenization rate led to the formation of emulsion gel with the most stability (90.075%) and the least aw (0.80%) and weight loss (4.403%).
The optimal values obtained from this step (2 gr RBO, 6.57 gr APS and 7 rpm homogenization rate) were combined with the different levels of natamycin and were used as an active edible coating in the second phase in order to study their effects on the chemical and sensory properties of grape.
The antimicrobial effect of natamycin
Microbial safety is one of the most important factors to be considered for the preservation of minimally processed foods [45].
This section was done in three steps. In the first step, spores, broth culture medium and Tween 80 were used. Combination of culture medium and Tween 80 were also selected as control. The absorbance was read immediately at a wavelength of 600 nm and 3 repetitions.
The second step was performed like the first stage, with the difference that natamycin was added to the formulation at 5 levels and the adsorption was also read immediately without incubation in three repetitions. The third step was the same as the previous step; but after preparing the plate, the incubation was done for 5 d at 25°C. Then, the absorption was read with the same conditions (at wavelength of 600 nm and 3 repetitions).
On the other hand, one stage of incubation of the 96 houses plate containing the Tween 80, culture medium and natamycin was also performed; but without the presence of spores as main reference for comparison with other plates (absorption 3). Diagrams of absorption changes of the mold spores in the presence and absence of natamycin, before and after incubation were shown in Figures 5 to 7.
Food spoilage hardly ever happens with the presence of 108 spores. However, a range of 104-108 spores were considered for a more extensive study on the antimicrobial performance of natamycin. The use of 10 ppm of natamycin could be to cause destruction the maximum amount of spores (108) (Figure 5). In some studies, this concentration of antimicrobial agent (10 mg L-1) has been known as the most suitable and maximum dilution of natamycin to prevent spoilage of fruits [28].
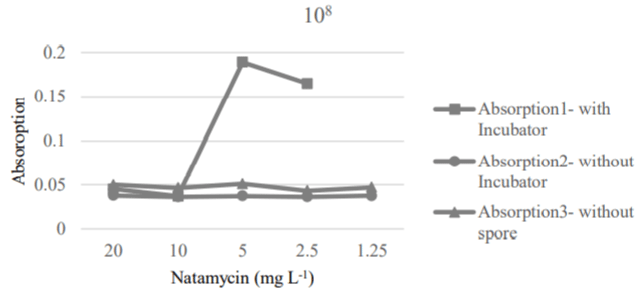
Figure 5: MIC and MFC values of natamycin for 108 spores.
The 4 and 2.5 mg L-1 concentrations of natamycin were effective to destroy the 106 and 104 of spores, respectively (Figure 6 and 7). These results showed that natamycin could be a good inhibitor and killer against the cause of corruption at the lowest level; therefore, it increased the shelf life of product.
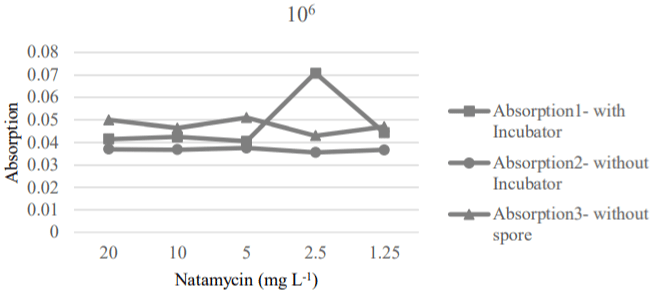
Figure 6: MIC and MFC values of natamycin for 106 spores.
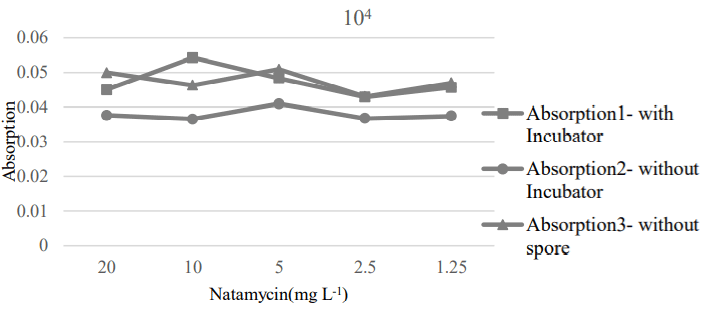
Figure 7: MIC and MFC values of Natamycin for 104 spores.
Analysis of grape’s chemical and sensory parameters
Analysis variance for the chemical parameters of grape after storage is presented in Table 6. The results indicated that both the concentrations of Natamycin and mold spore highly effected on the changes of grape’s characteristics during storage. The analysis of each parameter was discussed in the following.
Storage time
Source of changes
degree of freedom (df)
Sum of Squares (SS)
Mean Square (MS)
F
P
Second week
TA
6
0.021
0.004
181.41
0.000***
TSS
6
24.149
4.025
2.279
0.096ns
pH
6
0.323
0.054
26.741
0.000***
Weight loss
6
32.979
5.496
189068.41
0.000***
Spoilage percentage
6
3715.186
619.198
3139.705
0.000***
Fourth week
TA
6
0.011
0.002
27.270
0.000***
TSS
6
85.377
14.230
17.086
0.001**
pH
6
0.210
0.035
84.537
0.000***
Weight loss
6
915.556
152.593
2812.601
0.000***
Spoilage percentage
6
3057.462
509.577
9944.852
0.000***
Table 6: Results analysis variance for the chemical properties of grape’s clusters.
Reduce decay in grape: According to Figure 8, the highest corruption was observed at the sample control. The upward trend of spoilage percentage was continuing until the end of the second preservation period with the highest percentage of corruption in the 6 and control samples. The use of Natamycin at high concentrations greatly reduced the rate of spoilage of the coated samples during storage period [28,46]. The high concentration of antimicrobial substance (20 mg L-1) could prevent the spread of mold micelles on the fruit and its browning. Active coatings were caused descending trend on the growth of spoilage agent by reduced oxygen influence and mold spores contact with the surface of fruit [47,48].
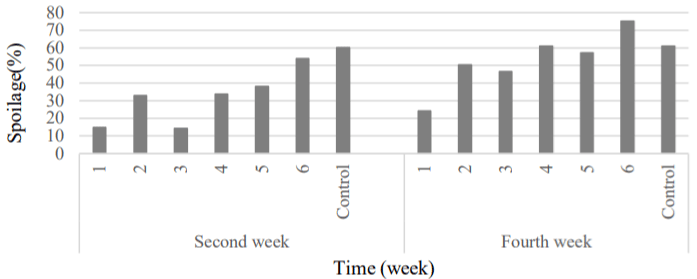
Figure 8: Comparison of fruit’s spoilage changes between samples were
coated by natamycin and Control.
1) APS (6.57gr) + RBO (2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr) +
RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) + 10 mg L-1
+ 106 spores; 4) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores; 5) APS
(6.57gr) + RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS(6.57gr) + RBO (2gr) +
2.5 mg L-1 + 104 spores; Control, APS (6.57gr) + RBO (2gr).
Weight loss: Water is the largest component of fruit and acts as a medium and chemical reactant during growth and postharvest storage. It is also very important to fruit texture. Significant difference was observed between the control and the coated samples with natamycin (Table 6). Weight loss due to transpiration during storage was observed for all treatments (Figure 9).
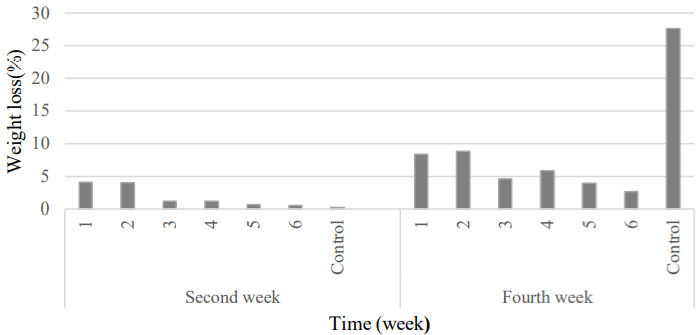
Figure 9: Comparison of fruit’s weight loss changes between samples were
coated by natamycin and Control.
1) APS (6.57gr) + RBO 438 (2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr)
+ RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr)+ RBO (2gr) + 10 mg
L-1 439 + 106 spores; 4) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores;
5) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr) + RBO
(2gr) + 2.5 mg L-1 + 104 spores; Control, APS (6.57gr) + RBO (2gr).
After two weeks of storage, the 2 and 1 samples had the highest weight loss; but at the end of the storage period, the control sample had the highest rate (27.62%) (Figure 9). Researchers found that the use of natamycin in the edible coating (containing rice bran oil which is one of the barriers to moisture loss) had great effect on preventing the weight loss of products [28,26]. Also, it was preserved the freshness of fruit and was reduced its waste after storage.
TSS: Total Soluble Solid (TSS) is an important parameter that affects fruit quality and consumer acceptability [49]. The results obtained from the TSS analysis are presented in Table 6. There weren’t observed significant difference between samples until the second week of storage. But the results were different at the end of storage period so that TSS was increased. The highest and lowest values were obtained in the 3 and control samples, respectively (Figure 10).
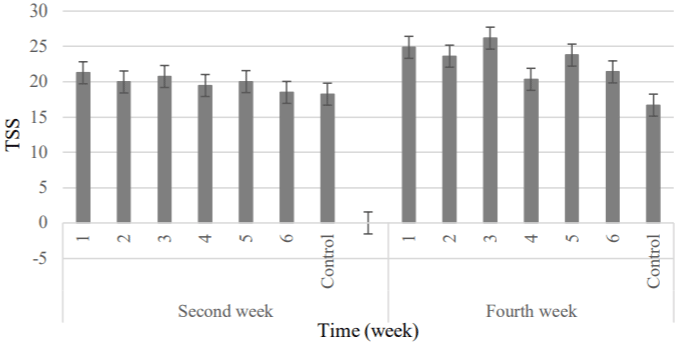
Figure 10: Comparison of fruit’s TSS changes between samples was coated
by natamycin and Control.
1) APS (6.57gr) + RBO (2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr) +
RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) + 10 mg L-1
+ 106 spores; 4) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores; 5) APS
(6.57gr) + RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr) + RBO (2gr) +
2.5 mg L-1 + 104 spores; Control, APS (6.57gr) + RBO (2gr).
Therefore, the use of natamycin at levels of 5 and 10 mg L-1 reduced the soluble solids and prevented the excessive ripening of fruit. About the TSS parameter, the natamycin concentration had the more effect than mold spores. Cong et al. [28] achieved the similar results.
TA: Changes in TA values of control and coated clusters are shown in Table 6. Coating had the significant effect on the titratable acidity at both storage periods (***p <0.001). So that, in the second week, the highest and lowest acidity were attributed to control and 3 samples, respectively. But, in the fourth week of storage, TA was decreased in some treatments (the lowest value was obtained for samples with the lowest concentration of natamycin) which could be due to delayed ripening of fruit texture by coatings. But this parameter significantly increased at control sample rather than other treatments (Figure 11).
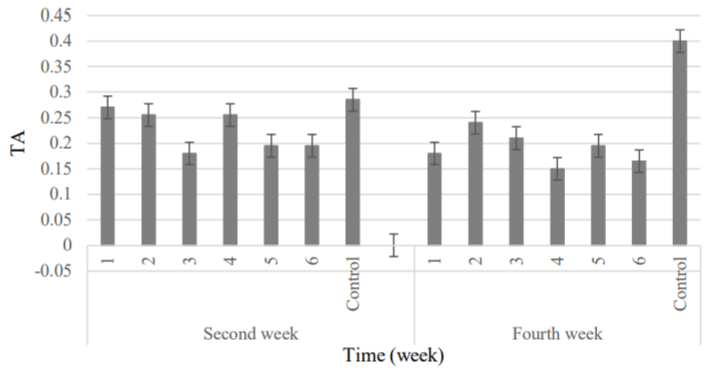
Figure 11: Comparison of fruit’s TA changes between samples was coated
by Natamycin and Control.
1) APS 471 (6.57gr) + RBO (2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr) +
RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) + 10 mg L-1
+ 106 spores; 4) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores; 5) APS
(6.57gr) + RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr) + RBO (2gr) +
2.5 mg L-1 + 104 spores; Control, APS (6.57gr) + RBO (2gr).
Among samples containing natamycin, the use of 10 mg L-1 of antimicrobial agent caused the better and more preservation of titratable acidity. Our results are consistent with Garcia et al. [44], El Ghuas et al. [50] and Cong et al.[28]; findings which showed natamycin improve the TA property of grape.
pH: The changes in the pH of control and coated clusters as a function of storage time is presented in Table 6. There wasn’t significant difference between the control and other treatments about pH until the second week of storage. But, the new results were obtained at the end of cold storage. The highest and lowest pH were related to the 1 and 6 samples, respectively (the second week) that agreed with the results of Cong et al. [28]; they researched the use of edible coating containing natamycin on increasing the shelf life of Hami-melon. The pH changes related to the fruit ripening [51]. In the fourth week, there wasn’t significant reduction in the pH values of treatments. The highest and lowest values of this parameter was attributed to the 5 and control samples, respectively (Figure 12).
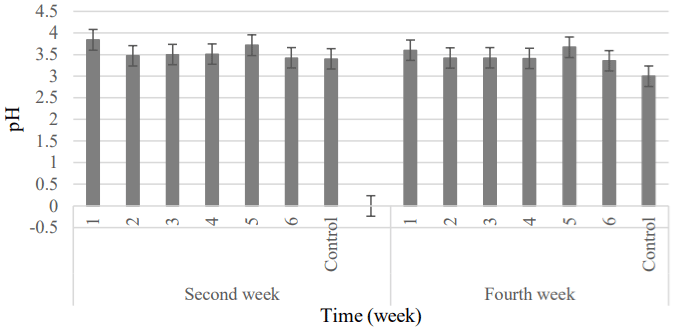
Figure 12: Comparison pH changes between samples were coated by
natamycin and Control.
1) APS (6.57gr) + RBO 491(2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr) +
RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) 492 + 10
mg L-1 + 106 spores; 4) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores;
5) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr) + RBO
(2gr) + 2.5 mg L-1 + 104 spores; Control, APS (6.57gr) + RBO (2gr).
Samples containing natamycin had the highest amount of pH compared to the control treatment. The results showed that value of this parameter increased with using the high concentration of natamycin in the coating.
Color: Results analysis of variance of the parameters a* (Greenness), b* (Yellowness) and L* (Luminosity) showed that significant changes were made in the parameters of greenness and brightness, but the coatings hadn’t significant effect on the yellowness of clusters (Table 7).
Storage time
Source of changes
degree of freedom (df)
Sum of Squares (SS)
Mean Square (MS)
F
P
Second week
a*
6
188.807
31.468
4.946
0.027*
b*
6
473.023
78.837
3.36
0.069ns
L*
6
1018.358
169.726
4.531
0.034*
Fourth week
a*
6
436.812
72.802
9.054
0.005**
b*
6
267.975
44.663
2.567
0.122ns
L*
6
1045.012
174.169
7.722
0.008**
Table 7: Results of analysis of variance related to the color parameters of grape’s clusters.
According to Figures 13 to 15, in the second week, the minimum and maximum greenness were attributed to the 5 and 1 samples, respectively. Regarding the parameter L*, the highest and lowest value was belonged to the 2 and 6 samples, respectively [28]. At the end of the refrigeration period, a* parameter had decreasing trend but the amount of yellowness didn’t significant change. The least amount was obtained in the control cluster that agreed with the results of study which was done on increase the yellowness parameter at presence of natamycin (the creamy powder) into the edible coating [52]. In the final week of storage, the 1 and 2 treatments showed the highest brightness; although, the lowest amount was belonged to the control sample. The high transparency of coated grapes was attributed to the hydrophobic and strong oxidizing structure of natamycin. This factor intensified the brightness of product due to the combination of high concentrations of natamycin with coating consisting of rice bran oil and acetylated potato starch (this type of starch produces the high transparency gel).
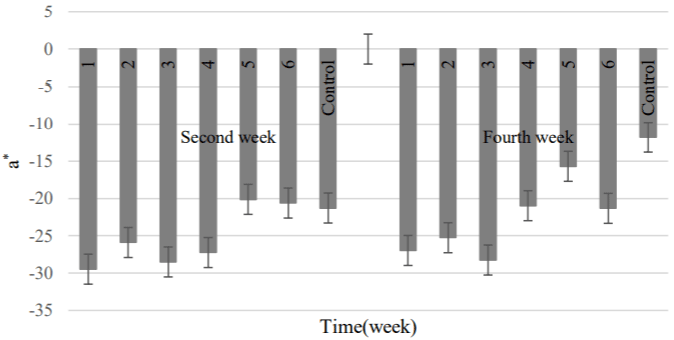
Figure 13: Changes treatments were studied about the color parameter a*.
1) APS (6.57gr) + RBO (2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr) +
RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) + 10 mg L-1
+ 106spores; 4) APS (6.57gr) + RBO (2gr) + 5 mg L-1 + 106 spores; 5) APS
(6.57gr) + RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr)+ RBO (2gr) +
2.5 mg L-1 + 104 spores; Control, APS (6.57gr) + RBO (2gr).
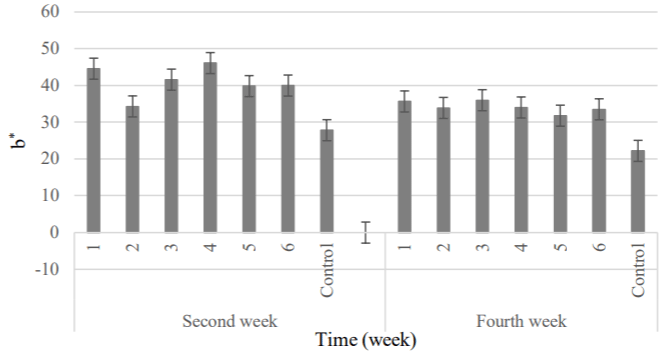
Figure 14: Changes treatments were studied about the color parameter b*.
1) APS (6.57gr) + RBO (2gr) + 20 mg L-1 + 108 spores; 2) APS (6.57gr) +
RBO (2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) + 10 mg L-1
+ 106 spores; 4) APS (6.57gr)+ RBO (2gr) + 5 mg L-1 + 106 spores; 5) APS
(6.57gr)+ RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr) + RBO (2gr) +
2.5 mg L-1 + 104 spores; Control, APS (6.57gr)+ RBO (2gr).
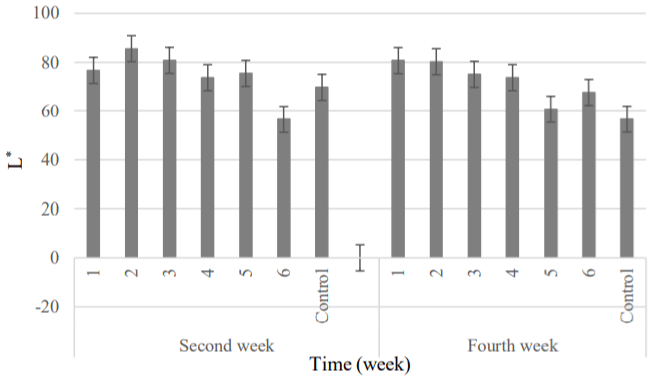
Figure 15: Changes treatments were studied about the color parameter L*.
1) APS (6.57gr) + RBO (2gr) + 20 mg L-1 +108 spores; 2) APS (6.57gr) + RBO
(2gr) + 10 mg L-1 + 108 spores; 3) APS (6.57gr) + RBO (2gr) + 10 mg L-1 + 106
spores; 4) APS (6.57gr)+ RBO (2gr) + 5 mg L-1 + 106 spores; 5) APS (6.57gr)
+ RBO (2gr) + 5 mg L-1 + 104 spores; 6) APS (6.57gr)+ RBO (2gr) + 2.5 mg
L-1 + 104 spores; Control, APS (6.57gr)+ RBO (2gr).
Sensory properties: From the evaluators point of the view, samples containing the high concentrations of Natamycin (especially the sample 1) got the highest score (5) in terms of all the sensory parameters, compared to the sample control which had not antimicrobial agent. The results were extremely similar to the sample control in the low dilutions of Natamycin (sample 6) (Table 8).
Parameters
Color
Aroma
Appearance
Texture
Overall acceptability
Samples
Time (day)
Time (day)
Time (day)
Time (day)
Time (day)
14
28
14
28
14
28
14
28
14
28
Control
1.71ab
2a
2ab
1.71a
1.71ab
2ab
1.86ab
2a
1.86ab
2a
20 mg L-1 +108
3.71d
3.57c
3.43c
3.57c
3.43d
3.43c
3.71e
3.71c
3.29c
3.71c
10 mg L-1 +108
2.43abc
2.71b
2.14ab
2.29ab
2.71cd
2.29ab
2.43bc
3.57c
2.71bc
3bc
10 mg L-1 + 106
2.57bc
2.71b
2.86bc
1.86ab
2.57c
1.86a
2.43cd
3.14bc
2.43bc
2.43ab
5 mg L-1 + 106
2.86c
2.57ab
1.71a
2ab
2.71cd
1.71a
4.43de
2.29a
2.57bc
2a
5 mg L-1 + 104
2.14abc
2.57ab
2.29ab
2.57b
2.14bc
2.71bc
2.14ab
3.86c
2.14ab
2.43ab
2.5 mg L-1 + 104
1.57a
2.43ab
1.43a
2.29ab
1.29a
1.71a
1.71a
2.57ab
1.43a
1.57a
Standard error
0.143
0.099
0.139
0.125
0.133
0.126
0.13
0.132
0.132
0.137
Dissimilar letters in a column are indicative significant differences (*p <0.05).
Table 8: Effect of edible coating in combination with the different levels of natamycin on the sensory characteristics of grape’s clusters.
Conclusion
Edible coatings due to the use of biodegradable, recyclable and edible in their production have become a useful alternative to synthetic films and coatings. Using the combination of 2 gr rice bran oil, 6.57 gr acetylated potato starch and stirring speed of 7 rpm was selected as the best treatment because it was leaded to coating with the most stability and the least aw and weight loss. Using the higher concentrations of 10 mg L-1 was able to prevent from the growth of Botrytis cinerea mold’s micelles on the berries. Edible coating based on the rice bran oil and acetylated potato starch in combination with natamycin improved the chemical properties of grape through reduced the oxygen transfer and controlled the moisture. Also, it increased the shelf life (more than 2 weeks) and marketability of fruit. However, it is going to study the active edible coating’s tissue changes during storage. In order to improve the performance of natamycin will be needed to evaluation the application of encapsulation and nano-capsulation technology about the use of natamycin along with edible coating including acetylated potato starch and rice bran oil compared to the effect of its free form on the qualitative parameters and shelf life of grape.
Declaration
Acknowledgements: The present work was funded by the Deputy of Research, Ferdowsi University of Mashhad, Iran. The financial supports are gratefully acknowledged.
Author contributions: Azin Omid Jeivan: Conceptualization (equal); writing-review & editing (equal); writing-original draft preparation (lead); visualization (lead); software (lead); resources (lead); investigation (lead); formal analysis (lead); data curation (lead). Masoud Taghizadeh: conceptualization (equal); funding acquisition (lead); project administration (lead); writing –review and editing (equal); supervision (equal). Masoud Yavarmanesh: supervision (equal); conceptualization (supporting).
References
- Tournas VH, Katsoudas E. Mold and Yeast flora in fresh berries, grapes and citrus fruits. Inter. Journal Food Microbiology. 2005; 105: 11-17.
- Nelson KE. Harvesting and handling California table grape for market. University California Bull. No. 1913. 1979: 1985.
- Snowdon AL. A color atlas of postharvest diseases and disorders of fruits and vegetables, General introduction and fruits. CRC Press, Inc. Boca Raton, Florida. 1990: 1.
- Smilanick JL, Harvey JM, Hartsell PL, Henson DJ, Harris CM, et al. Factors influencing sulfite residues in table grapes after sulfur dioxide fumigation. 1990; 41:131-136.
- Mustonen HM. The efficacy of a range of sulfur dioxide generating pads against Botrytis cinerea infection and on out-turn quality of Calmeria table grapes. Journal Experimental Agric. 1992; 32: 389-393.
- Karabulut OA, Smilanick JK, Franka M, Mansour M, Droby S. Near-harvest applications of Metschnikowia fruiticola, ethanol and sodium bicarbonate to control postharvest diseases of grape in Central California. Journal Plant Disease. 2003; 87: 1384-1389.
- Smilanick JL, Henson DJ. Minimum gaseous sulfur dioxide concentrations and exposure periods to control Botrytis cinerea. Crop Protection. 1992; 11: 535-540.
- Zahvi T, Cohen L, Weiss B, Schena L, Daus A, Kaplunov T, et al. Biological control of Botrytis cinerea, Aspergillus and Rhizopus rots on table and wine grapes on Israel. Journal Postharvest Biology and Technology. 2000; 20: 115-124.
- Mostofi Y, Dehestani-Ardakani M, Razavi H. The Effect of chitosan on post-harvest life increase and qualitative characteristics of grape cultivar shahroudi. Journal Food Science and Technology 2011; 8: 93-102.
- Baldwin EA. Use of edible coating to preserve pecans at room temperature. Journal Food Research International. 2006; 41: 188 -192.
- Mariniello L, Di Pierro P, Esposito C, Sorrention A, Masi P, Porta R. Preparation and mechanical properties of edible pectin-soy flour films obtained in the absence or 703 presence of transglutaminase. Journal Biotechnology. 2003; 102: 191-198.
- Quezada-Gallo JA, Debeaufort F, Callegarin F, Voilley A. Lipid hydrophobicity, physical state and distribution effects on the properties of emulsion based edible films. Journal Membrane Science. 2000; 180: 37-40.
- Rajabian M. Study of the effect of edible coating of potato starch containing mountain coconut essential oil and thyme on the microbial and chemical properties of chicken breast kept at refrigerator temperature. PhD Thesis in Food Health, Shahrekord University. 2018.
- Luallen TE. Starch as a functional ingredient. Journal Food Technology. 1985; 39: 59-63.
- Rogols S. Starch modifications: A view into the future. Journal Cereal Foods World. 1986; 31: 872 - 874.
- Luallen TE. Structure, characteristics, and uses of some typical carbohydrate food ingredients. Journal Cereal Foods World. 1988; 33: 924 - 927.
- Schierbaum F, Kettlitz B. Studies on rye starch properties and modification. Part III. Viscograph pasting characteristics of rye starches. Starch/Stärke 1994; 46: 2-8.
- Mari´a A Garci´a, MN Martino and Noemi´ E Zaritsky. Plasticized Starch- Based Agricultural Food Chemistry. 1998; 46: 3758-3767.
- Farayde MF, Silvia Maria M, Thiago C, José Ignacio V, Lucia Helena IM. Edible films and coatings based on starch/gelatin: film properties and effect of coatings on quality of refrigerated red crimson grapes. Journal Postharvest Biology and Technology. 2015; 109: 57-64.
- Debeaufort F, Peroval C, Despre D, Courthaudon JT, Voilley A. Arabinoxylan- Lipid-based edible films and coatings. Influence of drying temperature on film structure and functional properties. Journal Agricultural and Food chemistry. 2002; 50: 2423 - 2428.
- Grist DH. Rice, 5th edn (Longman, London, UK). 1985.
- Sala GT, Van Vliet MA, Cohen Stuart GA, Van Aken, F van de Velde. Deformation and fracture of emulsion-filled gels. Effect of oil content and deformation speed. Journal Food Hydrocolloids. 2009; 23: 1381-1393.
- Hassani F, Javanmard M, Grossiy F. Evaluation of shelf life of Kiwi fruit coated with whey protein concentrates and rice bran oil. Iranian. Journal Food Science and Technology Research. 2010; 6: 167-158.
- Lifen Z, Fusheng C, Penglong Z, Shaojuan L, Hongshun Y. Influence of rice bran wax coating on the physicochemical properties and pectin nanostructure of cherry tomatoes. Journal Food Bioprocess Technology. 2017; 10: 349-357.
- Nourbehesht N. Production and evaluation of low fat sausages using emulsion gel based on inulin and rice bran oil. M.Sc. Isfahan University of Technology. Faculty of Agriculture. 2019.
- Naidu AS. Antimicrobials from animals. In Natural Antimicrobials for the Natural Food Antimicrobial Systems. CRC Press, Boca Raton, FL. 2003: 265-294.
- Dzigbordi B, Adubofuor J, Faustina Dufie WM. The effects of different concentrations of natamycin and the point of addition on some physicochemical and microbial properties of vanilla-flavoured yoghurt under refrigerated condition. Journal International Food Research. 2013; 20: 3287- 3292.
- Cong F, Zhang Y, Dong W. Use of surface coatings with natamycin to improve the storability of hami melon at ambient temperature. Journal Postharvest Biology and Technology. 2007; 46: 71-75.
- Hondrodimou O, Kourkoutas Y, Panagou EZ. Efficacy of natamycin to control fungal growth in natural black olive fermentation. Journal Food Microbiology. 2011; 28: 621-627.
- Riku A Talja, H Hele´n, YH Roos and Jouppila K. Effect of various polyols and polyol contents on physical and mechanical properties of potato starch-based films. Journal Carbohydrate Polymers. 2007; 67: 288-295.
- Shaw NB, Monahan FJ, Ó Riordam ED, Ó Sullivan M. Effect of soya oil and glycerol on physical properties of composite WPI film. Journal Food Engineering. 2002; 51: 299-304.
- Baliga B, Madaiah N. Quality of Sausage emulsion prepared from mutton. Journal Food Science. 1970; 35: 383-385.
- Iranian National standard. Fruit and vegetable products- determination of acidity- Test method. Iranian Institute of Standards and Industrial Research (First ed). 1992: 373.
- Huaa Ch, Lib Y, Wanga X, Kaia K, Sua M, Shia W, et al. The effect of low and high molecular weight chitosan on the control of gray mold (Botrytis cinerea) on kiwi fruit and host response. Journal Science Horticulturae. 2019; 246: 700-709.
- Dos Santos NST, Athayde Aguiar AJA, de Oliveira CEV, de Melo e Silva CS, Sousa da Silva R and Stamford et al. Efficacy of the application of a coating composed of chitosan and origanum vulgare L. essential oil to control Rhizopus stolonifer and Aspergillus niger in grapes (Vitis labrusca L). Journal Food Microbiology. 2012; 32: 345-353.
- GAO P, Zhu Z, Zhang P. Effects of chitosan-glucose complex coating on postharvest quality and shelf life of table grapes. Journal Carbohydrate Polymers. 2013; 95: 371.
- Parvaneh V. Food quality control and chemical testing. Tehran University Press. 1986.
- Shirin S, Asghar R. Effect of natural aloe vera gel coating combined with calcium chloride and citric acid treatments on grape (Vitis vinifera L. Cv. Askari) quality during storage. Journal Food Science and Technology. 2014; 2: 1-5.
- Abdollahi A. Effect of three herbal essences on the control of fungal diseases after harvest of two grape varieties. Master Thesis. Urmia University. 2008.
- Ebrahimi B, Mohammadi R, Rouhi M, Mortazavian AM, Shojaee-Aliabad S, Koushki. Assessment of quality parameters. Journal Food Science and Technology, 2018; 87: 54-60.
- Jutamongkon R, Praditdoung S, Vananuvat N. Effect of rice bran waxing on fruit and vegetable storage. Journal Natural Science. 2011; 45: 1115 - 1126.
- Huang X, Kakodaand Y, Gui W. Hydrocolloid in emulsions particle size distribution and interfacial activity. Journal Food Hydrocolloids. 2001; 15: 533 - 542.
- Slavutsky MA, Bertuzzi AM. Formulation and characterization of nanolaminated starch based film. Journal Food Science Technology. 2015; 61: 407-413.
- Garcia MA, Martino MN, Zaritzky NE. Lipid addition to improve barrier properties of edible starch-based films and coatings. Journal Food Science. 2000; 65: 941-944.
- Wiley RC. Minimally Processed Refrigerated Fruits and Vegetables. Chapman and Hall, New York, USA. 2017.
- Durana M, Aday MS, Demirel Zorbaa NN, Temizkana R, Büyükcanb MB, Caneraa C. Potential of antimicrobial active packaging ‘containing natamycin, nisin, pomegranate and grape seed extract in chitosan coating’ to extend shelf life of fresh strawberry. Journal Food and bio products process. 2016; 98: 354 - 363.
- Gennadios A, Kurth LB. Application of edible coatings on meats, poultry and seafood’s: A review. Journal Lebensm Wiss Technology. 1997; 30: 337-350.
- Lee JY, Park, HJ, Lee CY, Choi WY. Extending shelf life of minimally Technology. 2003; 36: 323-329.
- Aday MS, Caner C. The shelf life extension of fresh strawberries using an oxygen absorber in the biobased package. Journal Food Science Technology. 2013; 52: 102-109.
- El Ghaouth A, Arul J, Ponnampalam R, Boulet M. Chitosan coating effect on storability and quality of fresh strawberries. Journal Food Science. 1991; 56: 1618-1620.
- Han C, Zhao Y, Leonard SW, Traber MG. Edible coatings to improve storability and enhance nutritional value of fresh and frozen strawberries (Fragaria × ananassa) and raspberries (Rubus ideaus). Journal Postharvest Biology and Technology. 2004; 33: 67-78.
- Carolina P Ollé Resa, Rosa J Jagus, Lía N Gerschenson. Effect of natamycin, nisin and glycerol on the physicochemical properties, roughness and hydrophobicity of tapioca starch edible films. Journal Materials Science and Engineering. 2014; 40: 281-287.