Abstract
Ankle proprioceptive impairments after ankle sprain appear to be in the origin of the neuromuscular dysfunction in Chronic Ankle Instability (CAI), however the analysis of this condition has been focused on a unilateral approach. Considering that proprioceptive information have a determinant role in ipsilesional limb responses but also the contralesional ones, the present study aims to analyze the bilateral Short Latency Responses (SLR) in response to an unilateral perturbation in individuals with CAI. Two groups of athletes participated in the present study. One was composed by participants presenting CAI (CAI group, n = 16), while the other was composed by participants not presenting ankle sprain episodes (control group, n = 20). The electromyographic activity of the Peroneal Brevis and Longus (PB and PL), Tibialis Anterior (TA) and Soleus (SOL) muscles was collected during a unilateral sudden inversion perturbation in both the support and perturbed limbs. The timing of muscle activation of both limbs was used for analysis. Delayed SLR of TA (p = 0.009) and in SOL (p = 0.042) muscles were observed in the contralesional limb of the CAI group in the support position compared to the control group. In CAI group, delayed SLR of PB (p = 0.023) and SOL (p = 0.004) muscles was found in the contralesional limb in the support position compared to the ipsilesional one. The rehabilitation of individuals with CAI should also be focused on the contralesional limb while assuming a support position and also, contralesional limb should not be considered a reference for comparison between limbs.
Keywords: Short latency responses; Chronic ankle instability; Postural control; Ankle
Introduction
Postural control involves a complex multisegmental process to maintain the dual purpose of orientation and stability [1-3]. From several sensory inputs, the proprioceptive information from the foot and ankle plays a central role in postural control [3-5]. It has been argued that one of the most common dysfunctions in athletes and active subjects, the Chronic Ankle Instability (CAI), is associated with changes in neuromuscular response, resulting from changes in the sensitivity of neuromuscular spindles in the ankle region that leads to a delayed muscle activation [6-8]. The CAI, defined as a subjective and repeated perception of giving away episodes has been estimated to occur in 40% to 70% of cases of lateral ankle sprain [6,8,9].
Despite several studies have been developed aiming to found the factors responsible for CAI, they were based on an unilateral approach [10,11]. The evidence demonstrating that the neuromuscular responses are influenced also from proprioceptive receptors of the contralesional limb turn relevant the study of bilateral neuromuscular responses in cases of unilateral ankle sprain episodes [6,12]. Most of the studies dedicated to CAI have been focused on studying the timing of short latency responses (SLR) in the sprained limb [10,11]. The bilateral evaluation of SLR would provide a better understanding of postural dysfunctions that contribute to CAI [6,13,14].
Considering the exposed, the objective of this study is to evaluate the bilateral SLR in response to an unilateral perturbation in individuals with CAI.
Considering that an unilateral perturbation in one lower limb leads to bilateral postural adjustments it can be hypothesised that individuals with unilateral CAI present delayed SLR in both injured and uninjured limbs [6].
Methods
Subjects
A total of thirty six athletes, aged between 18 and 30 years old, who practiced one of the modalities where the ankle ligament injury constituted the most prevalent lesion (soccer, basketball, volleyball and handball players) participated in the present study [15,16]. The participants were selected by a previous questionnaire and were evaluated in the Movement and Human Activity Center of Studies. The participants were divided into two groups according to the answers obtained in the questionnaire and the presence of CAI. The group with CAI was constituted by individuals with unilateral CAI (n=16) and the control group was composed by individuals with no history of ankle sprain (n=20) (Table 1).
The inclusion and exclusion criteria were based on the recommendations stated by the International Ankle Consortium [17]. The CAI group included individuals presenting: 1) history of unilateral sprain of degree II and III [18]; 2) functional ankle instability according to the Ankle Instability Instrument and/or; 3) mechanical instability identified through the Anterior Drawer Test, Prone Anterior Drawer and Talar Tilt test [7,19]. Subjects were excluded from both groups if they present: 1) history of surgery and/or ankle fracture; 2) deficits of the vestibular system; 3) other lower limb injuries in the last 3 months; 4) or presence of painful symptoms at the time of tests [10,20]. Participants that had the last sprain episode in the 3 months the preceded the study were excluded from the CAI group [18,20].
Variables
Statistic measures
Control group (n=20)
CAI group (n=16)
t
p
Age (years)
Min.
Max.
M ± SD18
27
21,84± 2,2118
28
20,54±2,65-1,713
0,94
Height (meters)
Min.
Max.
M ± SD1,55
1,9
1,78±0,091,60
1,94
1,76±0,09-0,716
0,478
Weight (Kg)
Min.
Max.
M ± SD48
90
73,79±11,5254
95
69,63±11,59-1,173
0,248
BMI (Kg/m2)
Min.
Max.
M ± SD17,57
26,87
22,22±2,1917,72
30,84
23,52±3,10-0,842
0,405
Sport
-
-
Football
-
14
10
Volleyball
-
5
3
Basketball
-
0
2
Handball
-
1
1
CAI type
Functional
Mechanical-
--
-8 (4 ND, 4D)
8 (4 ND, 4D)-
-
Degree of sprain
-
--
-II (n=14)
III (n=2)-
-
Last sprain (months)
Min.
Max.
M ± SD-
-
-3
18
10,53±3,96-
-
Caption: Minimum (Min.), Maximum (Max.), Mean (M), Standard Deviation (SD), p value (p), kilograms (Kg), Meters (M), Body Mass Index (BMI), Dominant limb (D), Non-Dominant limb (ND)
Table 1: Characterization of CAI group and control group.
Muscle
Anatomical references
TA
In the initial third of the line connecting the upper end of the tibia to the lower end of the medial malleolus.
SOL
Two centimeters distally to the lower edge of the medial gastrocnemius and two centimeters medially to the posterior line of the leg.
LP
¼ of the distance between the lateral malleolus and the lateral epicondyle
CP
¾ the distance between the lateral malleolus and the lateral epicondyle
Ground electrode
In the center of the patella
Table 2: Anatomic references for the placement of the electrodes.
The study was approved by the local ethics committee and implemented according to the Declaration of Helsinki. All individuals gave their written consent.
Instruments
The AII is an instrument that can accurately identify individuals with functional ankle instability [21]. This instrument is composed by 9 closed questions organized into three items: initial ankle sprain severity, history of instability and instability during activities of daily living [22]. Individuals that respond ‘yes’ in at least 4 questions were included in the present study. The Electromyography (EMG) signal of the Peroneal Brevis (PB), Peroneal Longus (PL), Tibialis Anterior (TA) and Soleus (SOL) muscles was acquired through a portable electromyograph bioPLUX research (PLUX® wireless biosignals SA, Arruda dos Vinhos, Portugal), with a frequency of acquisition of 1000 Hz through the software Monitor Plux. This system has a gain of 1000, 110dB Common Mode Rejection Ratio (CMRR), an input impedance >1Gohm and a passband of 25-500Hz. For each muscle two disposable, self-adhesive AgCl electrodes (Dahlhausen®, Köln, Alemanha) with a circular surface and 1 cm radius were used with a distance of 20mm between the detection surfaces. The signal was processed through software Acqknowledge® version 3.9 from Biopac Systems, Inc. The impedance of the skin was measured by an F(Noraxon®, Scottsdale, U.S.A.).
A tilt platform was used to force 30º of subtalar joint inversion. The platform included two movable plates (trapdoors) so that either foot could be tilted independently, thus removing any subject anticipatory effect. A triaxial accelerometer (bioPLUX research) connected to a wireless signal acquisition system was placed in each movable plate to detect the onset of the tilt mechanism (first deflection of the accelerometer signal).
Procedures
Skin preparation and placement of electrodes: Initially, the skin was prepared to reduce its impedance to values below 5000Ω [23]. The AgCl electrodes were positioned according to the anatomical references mentioned in Table 2. In the control group the electrodes were placed in the non-dominant limb [24]. In the individuals of the CAI group, the electrodes were placed in both limbs.
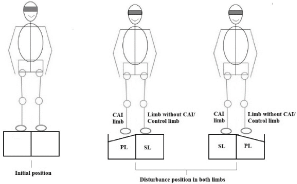
Figure 1: Schematization of the disturbance application in the individuals. Caption: PL - perturbed limb; SL - support limb.
Data acquisition: Both signals were acquired, synchronized and integrated with bioPLUX system. All individuals were asked to stand quietly with the support base aligned at shoulder width with one foot in each trapdoor, keeping their arms by their sides, and to focus on a target 2 meters away and at eye level during 30 seconds (Figure 1) [25]. After this period, one limb at a time was randomly exposed to the unilateral sudden inversion mechanism (30º of inversion) without the participant knowing the time and in each limb the perturbation would be applied (Figure 1) [26]. In each trial the trapdoor was randomly released by pushing a foot switch not visible for the subject. Six trials were performed: in 3 trials the perturbation was applied to the limb with CAI and in the remaining 3 trials the perturbation was applied in the contralesional limb. One minute of rest between each trial [27]. The order in which the disturbances were applied was randomized. The limb exposed to the sudden inversion perturbation was designated by perturbed limb while the contralesional limb was designated by support limb (Figure 1). The EMG activity of both limbs was collected in both positions [27]. Finally, the mean of each three trials was performed and this values were used for data processing.
Data processing: The EMG signal was full wave rectified and first order lowpass filtered (20 Hz) [28]. The moment of disturbance (T0) was identified as the first deflection of the accelerometer signal. The activation timing of SLR for each muscle was identified as the time instant, after T0, where a value equal to or greater than the baseline (identified as the interval from -450 to -500 milliseconds before T0) mean plus 3 times the standard deviation was found during at least 10 millisecond or when the time interval was smaller, but the response was followed by the Medium Latency Response (MLR) identified as a second peak [29,30].
Statistics
Statistical analysis was performed using IBM SPSS Statistics 22 software with a significance level of 0.05 [31]. The normality of the variables was assessed through the Shapiro-Wilk test [31]. The Independent Sample T-test (for samples that follow a normal distribution) and Mann-Whitney test (for samples that doesn’t follow a normal distribution) were used to compare the timing of SLR between control and CAI groups [31]. The Paired Sample T-test (for samples that follow a normal distribution) and Wilcoxon test (for samples that follow a normal distribution) were used to compare timing of SLR between ipsilesional and contralesional limbs in CAI group [31]. The non-parametric tests Mann-Whitney test (for the independent samples) and Wilcoxon (for the related samples) were used when the data didn´t followed a normal distribution and were market with an*.
Position
Muscle
Control Group
Limb
CAI Group
t
P
Mean
(ms)Standard
Deviation
(ms)Mean
(ms)Standard
Deviation
(ms)Support
TA
CAI
62
21,5
0,963
0,343
PB
61,9
23,3
-0,231
0,819
PL
62
21,2
0,416
0,680
SOL
61,9
21,3
-0,377
0,708
TA
49,3
24,4
Contra-lesional
67,6
12,4
2,816
0,009 *
PB
66,8
30,9
78,4
15,3
1,691
0,102
PL
56,2
22,9
63,4
18,5
0,940
0,355 *
SOL
64,3
22,4
78,3
20,8
2,126
0,042
Inversion
TA
CAI
51,3
19,2
0,141
0,889
PB
57
19,0
0,031
0,975
PL
54,8
23,6
0,560
0,580
SOL
61,5
21,4
0,140
0,889 *
TA
46
23,4
Contra-lesional
49,9
17,6
0,700
0,489
PB
54,6
24,8
55,6
28,6
0,152
0,881
PL
50,2
22,1
54
23,0
0,815
0,421
SOL
61,1
32,9
66,5
31,6
0,770
0,447
Table 3: Descriptive values of the timing of the SLR (ms) of the ipsilesional and contralesional limbs of CAI group and the control group, in the support and perturbed positions. P-values obtained from comparisons between groups are also presented. The variables where the non-parametric tests were used are marked with an *.
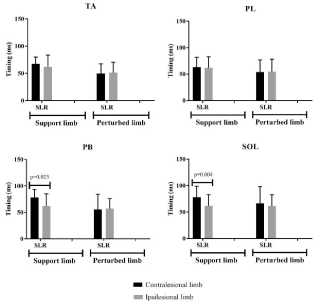
Figure 2: Proof values obtained from comparison of timing of SLR ipsilesional and contralesional limbs of the CAI group in the support and perturbed positions.
Results
The sample was composed by young football athletes with a normal body mass index. The proportion of individuals with mechanical instability and functional instability was the same. In the CAI group, the right limb was the most affected with a 2nd degree ankle sprain.
Statistically significant differences were found in the timing of TA and SOL SLR between the contralesional limb of CAI group and the control group in the support position. A delayed onset timing was observed in the contralesional limb, Table 3. In the support position statistical significant differences were also observed in PB and SOL SLR between contralesional and ipsilesional limbs of CAI group (Figure 2. A delayed SLR timing was observed in the contralesional limb compared to the ipsilesional limb (Figure 2).
Discussion
According to the literature, SLR alone can't perform the function of counteracting the forces and changes in the center of mass due to the disturbance, because of their spinal path that haven't sufficient magnitude to produce opposing forces to the instability [29,32,33]. However, these responses are the first that face of a disturbance and, depending on their duration and magnitude components, may be responsible for adapting the following responses, that are the MLR and Long Latency Responses (LLR), and therefore play an essential role in postural control [3,7].
The results indicate that individuals with CAI doesn´t present impairments in the SLR timings of ipsilesional limb, neither when it’s in support position nor in disturbance position, when compared to the control group. This information corroborate recent studies reporting that there are no impairments in SLR timings in the ipsilesional limb reinforcing the idea that the delay of SLR timings seem not to be directly associated with the CAI limb [5,20]. This fact is confirmed because the SLR timings between control group and CAI group are smaller than the 30 millisecond electromechanical delay, and also, SLR onset of both groups are in the range defined for the healthy population, that is, 40 milliseconds to 60 milliseconds after the disturbance [29,30]. Also, the findings show that SLR timings were not affected by the type of CAI.
Interestingly, the differences between groups occurred in the support position but between CAI contralesional limb and control group. The delayed SLR timings of TA and SOL in the contralesional limb, demonstrated postural control impairments in this limb [25,34,35]. Although not having a direct influence on the protection of the injury mechanism, this delay may increase the mechanical overload in the CAI limb during an inversion perturbation. In fact, an unpredictable inversion disorder causes a passive eversion of the subtalar joint, followed by active inversion, to counteract the initial disturbance in the support limb [25]. At the same time, the center of pressure undergoes an anterior translation, and this joint performs passive dorsiflexion, followed by active plantar flexion, opposing the mechanism of injury [25]. Thus, the action of the support limb contributes to recover postural stability [25]. Previous studies have demonstrated decreased postural stability in contralesional limb expressed through time-to-boundary in unipodal standing [36]. Considering the role of TA and SOL muscles in ankle dynamic stability, the results obtained seem to have relevant implications for postural stability [37]. However, it should be noted that only the non dominant limb of control group was evaluated. This aspect is a limitation from the present study as our results don´t enables to confirm if the limb dominance had any influence on the differences observed between groups. Future studies should explore if the lower limb dominance influence short latency postural responses in uninjured athletes.
The results of the present study also demonstrate that, in the support position, the contralesional member presents delayed SLR timings in PB and SOL compared to the ipsilesional limb. These findings corroborate the possibility of a reciprocal influence between limbs in cases of unilateral injury episodes [13,14,34,38]. In this perspective the contralesional limb should not be used as a reference for understanding the postural control dysfunction involved in CAI.
Conclusion
Subjects with CAI present, always when the limb is in support position, delayed SLR timings in TA and SOL of the contralesional limb when compared to uninjured subjects and delayed SLR timings in PB and TA of the contralesional limb when compared to ipsilesional limb. These findings have clinical implications, demonstrating that the rehabilitation of individuals with CAI should also be focused on the contralesional limb while assuming a support position.
References
- Lundy-Ekman L. Neurociência - Fundamentos para a reabilitação: Elsevier. 2008.
- Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice: LWW. 2011.
- Horak FB, Nashner LM. Central programming of postural movements: adaptation to altered support-surface configurations. Journal of Neurophysiology. 1986; 55: 1369-1381.
- Inglis JT, Horak FB, Shupert CL, Jones-Rycewicz C. The importance of somatosensory information in triggering and scaling automatic postural responses in humans. Experimental Brain Research. 1994; 101: 159-164.
- Munn J, Sullivan SJ, Schneiders AG. Evidence of sensorimotor deficits in functional ankle instability: a systematic review with meta-analysis. J Sci Med Sport. 2010; 13: 2-12.
- Hale SA, Hertel J, Olmsmsted-Kramer LC. The Effect of a 4-Week Comprehensive Rehabilitation Program on Postural Control and Lower Extremity Function in Individuals With Chronic Ankle Instability. Journal of Orthopaedic & Sports Physical Therapy. 2007; 37: 303-311.
- Brown C, Padua D, Marshall S, Guskiewicz K. Individuals with mechanical ankle instability exhibit different motion patterns than those with functional ankle instability and ankle sprain copers. Clin Biomech 2008; 23: 822-831.
- Hertel J. Functional Anatomy, Pathomechanics, and Pathophysiology of Lateral Ankle Instability. J Athl Train. 2002; 364-375.
- Mitchell A, Dyson R, Hale T, Abraham C. Biomechanics of ankle instability. Part 1: Reaction time to simulated ankle sprain. Medicine and science in sports and exercise. 2008; 40: 1515-1521.
- Hopkins JT, Brown TN, Christensen L, Palmieri-Smith RM. Deficits in peroneal latency and electromechanical delay in patients with functional ankle instability. J Orthop Res. 2009; 27: 1541-1546.
- Menacho MO, Pereira HM, de Oliveira BIR, Chagas LMP, Toyohara MT, Cardoso JR. The peroneus reaction time during sudden inversion test: Systematic review. Journal of Electromyography and Kinesiology. 2010; 20: 559-565.
- Riemann BL. Is there a link between chronic ankle instability and postural instability? Journal of athletic training. 2002; 37: 386-393.
- Nardone A, Grasso M, Giordano AMS. Different effect of height on latency of leg and foot short- and medium-latency EMG responses to perturbation of stance in humans. Neuroscience Letters. 1996; 206: 89-92.
- Di Giulio I, Maganaris C, Baltzopoulos V, Loram I. The proprioceptive and agonist roles of gastrocnemius, soleus and tibialis anterior muscles in maintaining human upright posture. J Physiol. 2009; 587: 2399-2416.
- Gutierrez G, Kaminski T, Douex A. Neuromuscular control and ankle instability. PM&R. 2009; 1: 359-365.
- Barone R, Macaluso F, Traina M, Leonardi V, Farina F, Di Felice V. Soccer players have a better standing balance in nondominant one-legged stance. American Journal of Sports Medicine. 2011; 2: 1-6.
- Gribble P, Delahunt E, Bleakley C, Caulfield B, Docherty C, Tik-Pui Fong D, et al. Selection Criteria for Patients With Chronic Ankle Instability in Controlled Research: A Position Statement of the International Ankle Consortium. Journal of Athletic Training. 2014; 49: 121-127.
- Hubbard TJ, Kaminski TW, Vander RA, Kovaleski JE. Quantitative assessment of mechanical laxity in the functionally unstable ankle. Medicine & Science in Sports & Exercise. 2004; 36: 760-766.
- Akhbari B, Takamjani I, Salavati M, Farahini H, Sanjari M. Ankle musculature latency measurement to varing angles of sudden external oblique perturbation in normal functionally unstable ankles. Medical Journal of The Islamic Republic of Iran. 2007; 20: 166-174.
- Eechaute C, Vaes P, Duquet W, Van Gheluwe B. Reliability and discriminative validity of sudden ankle inversion measurements in patients with chronic ankle instability. Gait & Posture. 2009: 30: 82-86.
- Pederson J. Investigating the Relationship Between FAI Questionnaires and Measures of Static and Dynamic Postural Stability. 2011.
- Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and Reliability of the Ankle Instability Instrument. Journal of Athletic Training. 2006; 40: 154-158.
- Merletti R. ISEK Standards for Reporting EMG Data 2010.
- Beynnon BD, Murphy DF, Alosa DM. Predictive Factors for Lateral Ankle Sprains: A Literature Review. J Athl Train. 2002; 37: 376-380.
- Mitchell A, Dyson R, Hale T, Abraham C. Biomechanics of ankle instability. Part 2: Postural sway-reaction time relationship. Medicine+ Science in Sports+ Exercise. 2008; 40: 1522-1528.
- Eils E, Rosenbaum D. A multi-station proprioceptive exercise program in patients with ankle instability. Medicine and science in sports and exercise. 2001; 33; 1991-1998.
- Hopkins J, McLoda T, McCaw S. Muscle activation following sudden ankle inversion during standing and walking. Eur J Appl Physiol. 2007; 99: 371-378.
- Fridlund AJ, Cacioppo JT. Guidelines for human electromyographic research. Psychophysiology. 1986; 23: 567-589.
- Petersen N, Christensen L, Morita H, Sinkjaer T, Nielsen J. Evidence that a transcortical pathway contributes to stretch reflexes in the tibialis anterior muscle in man. Journal of Physiology. 1998; 512: 267-276.
- Gutierrez GM. Neuromuscular Control in Ankle Instability. Pro Quest LLC. 2009; 1: 359-365.
- Marôco J. Análise Estatística com o SPSS Statistics: Report Number. Lda. 2011.
- Sinkjaer T, Andersen J, Nielsen J, Hansen H. Soleus long-latency stretch reflexes during walking in healthy and spastic humans. Clinical Neurophysiology. 1999; 110: 951-959.
- Taube W, Gruber M, Beck S, Faist M, Gollhofer A, Schubert M. Cortical and spinal adaptations indiced by balance training: correlation between stance stability and corticospinal ativation. Acta Physiologica. 2007; 189; 347-358.
- Sousa AS, Silva A, Tavares JM. Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: a review. . Somatosens Mot Res. 2012; 131-143.
- Delahunt E. Neuromuscular contributions to functional instability of the ankle joint. Journal of Bodywork and Movement Therapies. 2007: 203-213.
- Hertel J, Olmsted-Kramer L, Challis J. Time-to-Boundary Measures of Postural Control During Single Leg Quiet Standing. Journal of Applied Biomechanics. 2006: 67-73.
- Drake R, Mitchell A, Vogl W. Gray's anatomy fot students: Elsevier. 2005.
- Latash M. Control of human movement Champaign. Human Kinetics. 1993.
