Abstract
Neural coupling, frequently impaired in post-stroke subjects, can be assessed through upper limb flexor components against gravity like the brachioradialis muscles activity. Based on the known influence of the lower limb activity in the upper limb motor behavior it can be raised the question on whether the dysfunctional modulation of the extensors also happens in the brachioradialis muscle. The aim of this preliminary paper was to explore clinical evidence related with the dysfunctional modulation in the brachioradialis muscle along with the soleus muscle, expressed by an activation instead of inhibition pattern in APAs timeline, prior to stand to sit and gait, in stroke subjects. Six post stroke participants participated in the present study. The electromyographic activity of the soleus and brachioradialis muscles were recorded during stand to sit and gait initiation performance and was used to quantity muscle variation timing. The ground reaction forces assessed through a force pate were used to identify the beginning of the task. Post-stroke subjects presented in both functional tasks a pre-activation of soleus and braquioradialismuscles.Post stroke subjects with a dysfunction of soleus muscle modulation also showed a dysfunction in brachioradialis muscles, expressed by an activation pattern in both muscles prior to stand to sit and gait.
Introduction
The brain is a complex integrative network of functionally linked regions [1]. Stroke can be viewed as a disruption of an individual’s connectome caused by focal or wide spread loss of blood flow [2].
The postural control function is specifically dependent on the neural connectivity between the supplementary and premotor cortex to the reticular formation, through corticorreticular networks [3]. While the premotor and the supplementary motor area from cortex are responsible for anticipatory postural adjustments recruitment [4-7], the later has also been pointed as a possible cortical area related with the neural coupling between upper and lower limb [8,9].
This neural coupling is defined as “flexible, task-specific, physiologically meaningful linkage of limbs during complex movements” [10]. There is strong clinical evidence showing an atypical coupling between upper and lower limbs in post-stroke subjects [11], characterized in the upper limb by flexor components [11] related with the antigravity role of the brachioradialismuscle [12]. The known influence of the lower limb activity in the upper limb motor behavior [13] may raise the question on whether the lack of modulation of the extensors [specifically the soleus muscle] observed in post stroke subjects [14-16], also happens in the brachioradialis, considered its role in postural control of upper limb.
Functional tasks like stand to sit and gait initiation are characterized by a mechanical posterior displacement of center of pressure in the base of support [17-19] that is accompanied by the extensor motor neurons modulation prior to a flexor component activation through anticipatory postural adjustments. Specifically, these are expressed by the decrease of soleus activity prior to tibialis anterior activation and allows the release of the flexor component at the elbow joint, which is uncommon in stroke subjects. The modulation of this extensor response is related to the variation of the afferent proprioceptive input that reaches the cerebellum influencing the reticular formation [20-22], and vestibular nucleus [23]. Therefore, both the spino-cerebellum-reticular and spino-cerebellum-vestibular are neural circuits relevant in the variation of the muscular activity to extensor modulation.
Also, the ability to regulate the activity of anatomically distant muscles but functionally "coupled" within the scope of regulation of the extensor response is also based on the fact that an input of a segment is capable of influencing its adjacent segmental circuit, through spinal network [24]. Therefore, it can be hypothesized that post stroke subjects with dysfunctional soleus modulation, (expressed by an activation instead of an inhibition in APAs timeline, prior to stand to sit and gait) would also present dysfunction of brachioradial is modulation.
The main purpose of this preliminary paper was to explore clinical evidence (expressed by the muscular activity variation) related with modulation dysfunction in the brachioradialis along with the soleus muscles, in APAs recruitment prior to stand to sit and gait, in post stroke subjects (Figure 1).
According to Figure 1 all the participants showed an activation of the braquiorradialis muscle both sit to stand to sit and gait initiation during the time window of APAs.
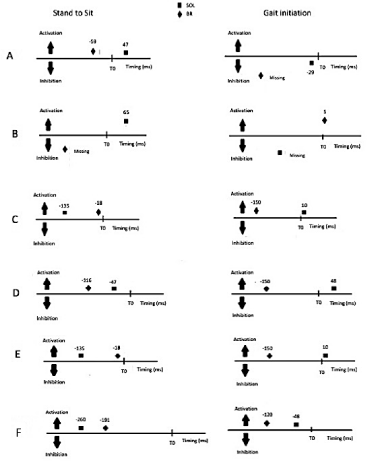
Figure 1: Illustrative scheme of the timing of activation of the solear and braquiorradialis muscles and their behaviour in the movement sequences of stand to sit and beginning of gait in participants A, B, C, D, E and F.
According to Figure 1 all the participants showed an activation of the braquiorradialis muscle both sit to stand to sit and gait initiation during the time window of APAs.
Methods
Participants
The present study is a case series study which included six participants (Table 1) from a private physiotherapy office, with a unilateral stroke in the territory irrigated by the MCA (confirmed by brain computerized axial tomography), in a sub-cortical level. Other inclusion criteria were the capacity to sit to stand to sit and gait initiation. Also, participants needed to present a dysfunction of the modulation of the soleus muscles (expressed by an activation in the temporal line of APAs recruitment). The exclusion criteria were other diseases that may affect the performance of the functional tasks [25,26] and a score in the Montreal Cognitive Assessment (MoCA) less than 26 points [27].
Instruments
Evaluation scale: To evaluate the cognitive status of the participants, the Montreal Cognitive Assessment (MoCA) instrument was used. It has high internal consistency (α Cronbach = 0.92), excellent temporal stability of the results, with test-retest r = 0.85 [p <.01; 33.47 [± 14.65] days] [27]. It has proven to be a valid, reliable, sensitive and accurate measure in assessing the cognitive impairment of individuals with stroke sequelae [28].
Surface Electromyography (sEMG): BioPlux Research (Plux® Lda., Portugal) device was used for recording surface Electromyography (EMG). For the recording, Silver Chloride (AgCl) Dahlausen 505 adhesive electrodes, of 10 mm size and circular shape, with a bipolar configuration and 20 mm distance between the two detection surfaces were used [29,30]. The skin impedance was measured using the Noraxon® meter (Noraxon, Scottsdale Arizona) [29]. EMG signals were analyzed in Analysis Software Acqknowledge® version 3.9 (sampling: 1000 Hz) (BIOPAC Systems, Inc., Goleta, USA).
Force plate: To record the ground reaction forces two Bertec® 600 mm long, 400 mm wide, Bertrand force plates were used (Bertec Corporation, model FP4060-10 and FP4060- 08, with headquarters at 6185 Huntley Road, Suite B, Columbus, OH 43229, USA). The force plates were connected to a Bertec AM 6300 amplifier and were used with a sampling frequency of 100 Hz. The amplifier was connected to a 16-bit analog / digital converter (Biopac). The reliability of the force plate presents an ICC?0,90. Strength values were normalized according to the weight of each subject [31]. Data were acquired through Qualisys Track Manager (Qualisys AB, based in Packhusgatan 6, Gothenburg – Sweden) [18] and the obtained signals were processed through Acqknowledge Software, version 3.9.0 (BIOPAC Systems, Inc., Goleta, USA).
Participant
Gender
Age
Weight
Height
Stroke time
CONTRA side
Areaofinjury
A
M
61
89
176
6
Left
Lenticulocapsular interna anterior
B
M
47
95
176
30
Right
Lenticulo-capsular and corona radiata
C
M
37
76
185
30
Left
Cortico-subcortical
D
F
54
75
158
90
Left
Fronto-parietal and insular
E
M
34
86
185
18
Left
Lenticulocapsular
F
F
57
65
160
12
Right
Striatocapsular
Table 1: Characterization of the participants [gender, male [M] and female [F], age [years], weight [kg], height [cm], evolution time [months], CONTR side.
Procedures
Before the collection of EMG, the skin was prepared to guarantee an impedance less than or equal to 5KΩ. The placement of the electrodes in the soleus and Medial Gastrocnemius (MG) muscles respected the European orientations of SENIAM [32]. The activity of MG was recorded to ensure the correct placement of electrodes in the soleus muscle. For the brachioradialis muscle, the electrodes were placed in the muscle belly, 4 cm below the lateral epicondyle of humerous, in the antero-lateral face of the forearm.
Both functional tasks were initiated with one foot on each [33] force plate and with the upper limbs along of the body, keeping the eyes orientated to a specific signal 2 m away [34] during 60 seconds [35]. Previous to the verbal command, the participants were instructed to perform the task without using the upper limbs and changing the feets between repetitions [36]. The tasks were performed with the participants using usual footwear [37] (ensuring that the same footwear was used in the two recording moments) and at a self-selected velocity. Three valid repetitions were performed with a minute of rest between. EMG signal was recorded in both limbs simultaneous.
Data processing and analysis
The center of pressure data was low-pass filtered using a fourth-ordered Butterworth filter by using a zero-phase lag with a cutoff frequency of 20 Hz. The identification of the beginning of the stand to sit and gait initiation (T0) was based on the anteroposterior component of the center of pressure. In both tasks, T0 was defined as the beginning of the interval lasting at least 50 ms during which the value of the anteroposterior component of center of pressure was superior to the mean of its basal value plus three standard deviations [18,38].
The electromyographic signals were filtered using a zero-lag, second-order Butterworth filter with an effective band pass of 20 to 450 Hz, and the root mean square was calculated. The muscle latency was detected in a time window from −450 to +50 ms in relation to T0. The latency for a specific muscle was defined as the instant lasting for at least 50 ms when its EMG amplitude was higher (activation) or lower (inhibition) than the mean of its baseline value plus 3 Standard Deviation (SD), measured from −500 to −450 ms.
Discussion
The main purpose of this preliminary paper was to explore clinical evidence (expressed by the muscular activity variation) related with the dysfunction of modulation in the Brachioradialis muscle along with the soleus muscle, in corporating an a typical neural coupling during stand to sit and gait initiation, in stroke subjects.
The importance of the extensor motor neuron modulation has been already demonstrated, especially in the ankle joint during APAs recruitment [18]. Despite this well-known mechanism involving ankle strategy, and more specifically the soleus muscle modulation, there is no evidence about this processes in upper limb antigravity muscles, such as brachioradialis. The importance of assessing this muscle is related with the clinical evidence of stroke patients presenting an upper limb flexor pattern associated with pronation. In fact, these patients often present a stereotyped behavior of brachioradialis muscle characterized by an invariable component of tension/length.
As post stroke subjects present a sub-cortical lesion, they may have compromised the SMA output which justifies the dysfunctional APAs behavior found in this study. In fact, as SMA outputs are mainly towards reticular formation (medullarys) [39] and reticulospinal axons branch extensively within the spinal cord, contacting many motoneurons pools [40], they may influence motoneurons projecting both to proximal and distal limb muscles [41,42], justifying the atypical behavior of distal muscles such as the one explored in this study.
Exploring this possible neural coupling between upper and lower limbs is also justified by the knowledge that interlimb coordination between the arms and legs may play an important role in the recovery of stable walking [43].
Moreover, this study may have contributed to the awareness for the need of the health organizations to search therapeutic strategies that respect the concept of neuralconnectivity in order to potentiate the maximal neuro-motor recovery [44]. Specifically. both soleus and brachioradialis are monoarticular muscles should be considered in therapeutic strategies since they are more susceptible to sensory adaptation than biarticular muscles [45]. As the afferent feedback plays a dominant role in mediating interlimb reflexes [46] it is important to consider that the input through lower limbs also can influence motor output of the upper limb. In this way, this clinical reasoning and intervention strategies are in agreement with the current state of the art of looking to the individual with stroke in a holistic perspective.
However, there are some limitations related to the analysis of this behavior in healthy population during these functional tasks. The need of assessing this neural coupling in healthy population is also justified by the neurological assessment and intervention which needs to be looked in a global and interconnected body segments with neural and biomechanics interplay.
Conclusion
Post stroke subjects with a dysfunction of soleus muscle modulation also showed a dysfunction in brachioradialis muscles, expressed by an activation pattern in both muscles prior to stand to sit and gait.
Data Availability
The data used to support the findings of this study are included with in the article.
References
- Li Y, Wang D, Zhang H, Wang Y, Wu P, Yang Y, et al. Changes of Brain Connectivity in the Primary Motor Cortex After Subcortical Stroke: A Multimodal Magnetic Resonance Imaging Study. Medicine [Baltimore]. 2016; 95: e2579.
- Silasi G, Murphy TH. Stroke and the connectome: how connectivity guides therapeutic intervention. Neuron. 2014; 83: 1354-1368.
- Lemon RN. Descending pathways in motor control. Annu Rev Neurosci. 2008; 31: 195-218.
- Chang WH, Tang PF, Wang YH, Lin KH, Chiu MJ, Chen SH. Role of the premotor cortex in leg selection and anticipatory postural adjustments associated with a rapid stepping task in patients with stroke. Gait Posture. 2010; 32: 487-493.
- Yoshida S, Nakazawa K, Shimizu E, Shimoyama I. Anticipatory postural adjustments modify the movement-related potentials of upper extremity voluntary movement. Gait Posture. 2008; 27: 97-102.
- Jacobs JV, Lou JS, Kraakevik JA, Horak FB. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience. 2009; 164: 877-885.
- MacKinnon CD, Bissig D, Chiusano J, Miller E, Rudnick L, Jager C, et al. Preparation of anticipatory postural adjustments prior to stepping. J Neurophysiology. 2007; 97: 4368-79.
- Arya KN, Pandian S. Interlimb neural coupling: implications for poststroke hemiparesis. Ann Phys Rehabil Med. 2014; 57: 696-713.
- Debaere F, Swinnen SP, Béatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage. 2001; 14: 947-958.
- Dietz V, Schrafl-Altermatt M. Control of functional movements in healthy and post-stroke subjects: Role of neural interlimb coupling. Clin Neurophysiol. 2016; 127: 2286-2293.
- Kline TL, Schmit BD, Kamper DG. Exaggerated interlimb neural coupling following stroke. Brain. 2007; 130: 159-169.
- Holmes MW, Keir PJ. Muscle contributions to elbow joint rotational stiffness in preparation for sudden external arm perturbations. J Appl Biomech. 2014; 30: 282-289.
- Balter JE, Zehr EP. Neural coupling between the arms and legs during rhythmic locomotor-like cycling movement. J Neurophysiol. 2007; 97: 1809-1818.
- Silva A, Sousa AS, Tavares JM, Tinoco A, Santos R, Sousa F. Ankle dynamic in stroke patients: agonist vs. antagonist muscle relations. Somatosens Mot Res. 2012; 29: 111-116.
- Silva A, Sousa AS, Pinheiro R, Tavares JM, Santos R, Sousa F. Soleus activity in post-stroke subjects: movement sequence from standing to sitting. Somatosens Mot Res. 2012; 29: 71-76.
- Cheng PT, Chen CL, Wang CM, Hong WH. Leg muscle activation patterns of sit-to-stand movement in stroke patients. Am J Phys Med Rehabil. 2004; 83: 10-16.
- Silva A, Sousa AS, Pinheiro R, Ferraz J, Tavares JM, Santos R, et al. Activation timing of soleus and tibialis anterior muscles during sit-to-stand and stand-to-sit in post-stroke vs. healthy subjects. Somatosens Mot Res. 2013; 30: 48-55.
- Sousa AS, Silva A, Santos R. Ankle anticipatory postural adjustments during gait initiation in healthy and post-stroke subjects. Clin Biomech [Bristol, Avon]. 2015; 30: 960-965.
- Sousa AS, Silva A, Tavares JM. Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: a review. Somatosens Mot Res. 2012; 29: 131-143.
- Jiang J, Azim E, Ekerot CF, Alstermark B. Direct and indirect spino-cerebellar pathways: shared ideas but different functions in motor control. Front Comput Neurosci. 2015; 9: 75.
- Alstermark B, Ekerot CF. The lateral reticular nucleus: a precerebellar centre providing the cerebellum with overview and integration of motor functions at systems level. A new hypothesis. J Physiol. 2013; 591: 5453-5458.
- Brodal P. Further observations on the cerebellar projections from the pontine nuclei and the nucleus reticularis tegmenti pontis in the rhesus monkey. J Comp Neurol. 1982; 204: 44-55.
- Jörntell H. Cerebellar physiology: links between microcircuitry properties and sensorimotor functions. J Physiol. 2017; 595: 11-27.
- Meyns P, Bruijn SM, Duysens J. The how and why of arm swing during human walking. Gait Posture. 2013; 38: 555-562.
- Kusoffsky A, Apel I, Hirschfeld H. Reaching-lifting-placing task during standing after stroke: Coordination among ground forces, ankle muscle activity, and hand movement. Archives of physical medicine and rehabilitation. 2001; 82: 650-660.
- Prange G, Jannink M, Stienen A, van der Kooij H, Ijzerman M, Hermens H. An explorative, cross-sectional study into abnormal muscular coupling during reach in chronic stroke patients. Journal of NeuroEngineering and Rehabilitation. 2010; 7: 14.
- Freitas S, Simões MR, Martins C, Vilar M, Santana I. Estudos de adaptação do Montreal Cognitive Assessment [MoCA] para a população portuguesa. Avaliação Psicológica. 2010; 9: 345-357.
- Dong Y, Sharma VK, Chan BP-L, Venketasubramanian N, Teoh HL, Seet RC, et al. The Montreal Cognitive Assessment [MoCA] is superior to the Mini-Mental State Examination [MMSE] for the detection of vascular cognitive impairment after acute stroke. Journal of the Neurological Sciences. 2010; 299: 15-18.
- Correia PP, Mil-Homens P. A Electromiografia no Estudo do Movimento Humano. Lisboa2004.
- Matias R, Batata D, Morais D, Miguel J, Estiveira R. Estudo do Comportamento Motor dos Músculos Deltóide, Trapézio, e Grande Dentado Durante a Elevação do Braço em Sujeitos Asssintomáticos. EssFisioOnline. 2006; 2: 3-23.
- Turns LJ, Neptune RR, Kautz SA. Relationships between muscle activity and anteroposterior ground reaction forces in hemiparetic walking. Archives of Physical Medicine and Rehabilitation. 2007; 88: 1127-1135.
- SENIAM. Surface ElectroMyoGraphy for the Non-Invasive Assessment of Muscles Netherlands. 1999.
- Caderby T, Yiou E, Peyrot N, Bonazzi B, Dalleau G. Detection of swing heel-off event in gait initiation using force-plate data. Gait & Posture. 2013; 37: 463-466.
- Genthon N, Vuillerme N, Monnet JP, Petit C, Rougier P. Biomechanical assessment of the sitting posture maintenance in patients with stroke. Clin Biomech [Bristol, Avon]. 2007; 22: 1024-1029.
- Perlmutter S, Lin F, Makhsous M. Quantitative analysis of static sitting posture in chronic stroke. Gait Posture. 2010; 32: 53-56.
- Dubost V, Beauchet O, Manckoundia P, Herrmann F, Mourey F. Decreased trunk angular displacement during sitting down: an early feature of aging. Phys Ther. 2005; 85: 404-412.
- Kim MH, Yi CH, Yoo WG, Choi BR. EMG and kinematics analysis of the trunk and lower extremity during the sit-to-stand task while wearing shoes with different heel heights in healthy young women. Hum Mov Sci. 2011; 30: 596-605.
- Bishop M, Brunt D, Pathare N, Ko M, Marjama-Lyons J. Changes in distal muscle timing may contribute to slowness during sit to stand in Parkinsons disease. Clin Biomech [Bristol, Avon]. 2005; 20: 112-117.
- Li S, Francisco GE. New insights into the pathophysiology of post-stroke spasticity. Front Hum Neurosci. 2015; 9: 192.
- Baker SN. The primate reticulospinal tract, hand function and functional recovery. J Physiol. 2011; 589: 5603-5612.
- Riddle CN, Edgley SA, Baker SN. Direct and indirect connections with upper limb motoneurons from the primate reticulospinal tract. J Neurosci. 2009; 29: 4993-4999.
- Soteropoulos DS, Williams ER, Baker SN. Cells in the monkey ponto-medullary reticular formation modulate their activity with slow finger movements. J Physiol. 2012; 590: 4011-4027.
- Marigold DS, Misiaszek JE. Whole-body responses: neural control and implications for rehabilitation and fall prevention. Neuroscientist. 2009; 15: 36-46.
- Kleim JA. Neural plasticity and neurorehabilitation: teaching the new brain old tricks. J Commun Disord. 2011; 44: 521-528.
- Schindler-Ivens S, Brown DA, Brooke JD. Direction-dependent phasing of locomotor muscle activity is altered post-stroke. J Neurophysiol. 2004; 92: 2207-2216.
- Stevenson AJ, Geertsen SS, Sinkjær T, Nielsen JB, Mrachacz-Kersting N. Interlimb communication following unexpected changes in treadmill velocity during human walking. J Neurophysiol. 2015; 113: 3151-3158.
