Abstract
A middle-aged man had a silicone Great toe implant with a protective grommet placed for a painful right bunion deformity with degenerative joint disease.
Ten years later he had developed adult onset insulin dependent diabetes mellitus and many other comorbidities. His right Great toe area had become very grossly swollen and painful. The medial surgical incision broke down and began draining.
At surgery a broken implant and grommet were removed. The soft tissues were immense and were minimally debulked. Tissue histology and cultures were performed.
A polymicrobal infection was present and many silicone wear particles were present in the microscopic sections.
Intravenous antibiotics were given which resulted in a painless, but grossly enlarged medial foot.
Keywords: Silicone implant; Silastic®; adult onset; Insulin dependent diabetes mellitus; wound infection; wear particles
Introduction
Silicone joint implants were introduced in the early 1970’s by Albert Swanson, M.D. They have been used since that time in the orthopedic and podiatric communities for the management of joint deformity and arthritic conditions.
Initially, it was assumed that these implants were biocompatible, even when used in weight-bearing applications.
However, adverse effects such as synovitis, infection, bony necrosis, medullary and cortical destruction, and foreign body reactions often developed. Many of these negative processes were related to gross displacement of the implant and micro-particulate wear matter dispersion. The result of this can be lymphatic reticular transport of this silicone debris to local, and regional lymphatic tissues including lymph nodes and the surrounding tissues.
We present a case of massive lymphatic reaction to a silicone implant (Silastic®, Dow Corning, Midland, MI) in the first metatarsophalangeal joint (MTP), which was implanted ten years previously (Figure 1). Indications for the implanted prosthesis were hallux valgus with degenerative joint disease. The patient’s foot had massive lymphedema with fungating overgrowth of the affected foot and ankle soft tissues.
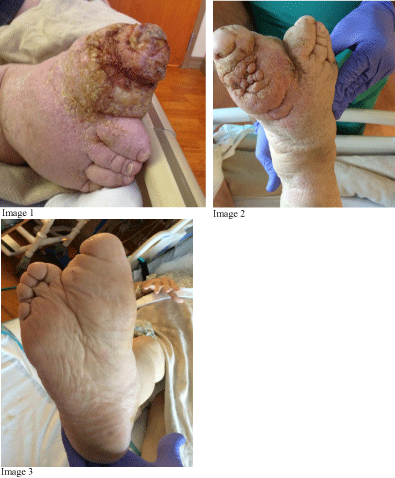
Figure 1: Right foot before (Image 1) and after removal of implant, 6 mos. previously (Images 2 and 3).
Case Report
The 63-year-old male presented as an inpatient for acute on chronic kidney failure and hyperglycemia. He complained of a painful, draining wound at the site of a prior right medial 1st MTP incision. Medical comorbidities included insulin-dependent diabetes mellitus, anemia, chronic obstructive pulmonary disease (COPD), chronic kidney disease, coronary artery disease (CAD), Crohn’s disease, sarcoidosis, peripheral neuropathy, hypertension and obesity.
Based on his x-rays, MRI and labs, the diagnosis of a chronic right foot infection with osteomyelitis associated with a 1st MTP Silastic® implant and grommet was made (Figure 2).
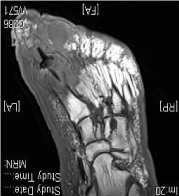
Figure 2: Pre-operative MRI.
The patient underwent an incision and drainage through the previous incision. A broken implant and the grommet were removed. The cavitary areas were curetted to the remaining bone. Tissue pathology and cultures and sensitivities of the deep tissues were obtained.
Pathology showed synovial tissue with areas of necrosis, granulation tissue, acute inflammation and foreign body giant cell reaction. The cultures showed a polymicrobial infection, which included vancomycin-resistant Enterococcus and methicillinresistant Staphylococcus aureus. The patient was treated with normal post-operative wound care and intravenous antibiotics for 6 weeks. The patient was lost to follow-up upon discharge.
Six months later he was re-hospitalized for hyperglycemia and a right foot wound. X-rays and an MRI were obtained (Figure 3). The patient stated he had no pain in the right foot, was able to walk short distances and desired to avoid further surgical intervention. The small medial wound was treated with local wound care. He was again lost to follow-up.
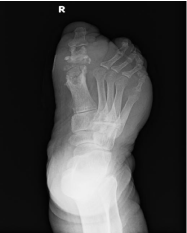
Figure 3: Post-operative x-ray.
Conclusion
There are numerous articles and case reports of complications associated with silicone implants in the upper and lower extremities [1-5]. This case showed a massive local and lymphatic response to an infected Silastic® implant with major overgrowth of the soft tissues not previously reported.
Local destruction of bone and soft tissues as well as a regional soft tissue reaction and enlargement is likely due to a silicone debris reaction. Silicone debris can be generated from abrasive wear at the bone-implant interface at the joint level as well as pistoning of the stems within the medullar canals of the respective bones. Attempts to decrease this wear by the addition of a grommet to the implant did not decrease this pathologic process. The lymphoreticular system can then transport these particulates proximally causing lymphedema and swelling. Locally, the soft tissues and synovial tissues can react, causing bony destruction by a macrophage-mediated lysosomal process. This pathophysiology is well-documented; however, the impressive enlargement and fungating nature of the soft tissues in this case differ from other reports in the literature associated with these implants. Perhaps the patient’s comorbidities contributed to the exaggerated response seen above.
References
- Fellander-Tsai L, Reinholt F, Turan I. Complications with infection and foreign body reaction after silicon implant arthroplasty in the second metatarsophalangeal joint in an adolescent: A case report. Journal of Foot and Ankle Surg. 1997; 36: 452-456.
- Freed JB. The increasing recognition of medullary lysis, cortical osteophytic proliferation, and fragmentation of implanted silicone polymer implants. Journal of Foot and Ankle Surgery. 1993; 32: 171-177.
- Hetherington V, Mercado, Karloc L, Grillo J. Silicone implant arthroplasty: a retrospective analysis. Journal of Foot and Ankle Surg. 1993; 32: 430-433.
- Patel D, Frascone S, DeLuca A. Synovitis secondary to silicone elastomeric joint implant. Journal of Foot and Ankle Surgery. 1994; 33: 628-632.
- Swanson A, Lumsden R, Swanson G. Silicone implant arthroplasty of the great toe. A review of single stem and flexible hinge implants. Clinical Orthop Relat Res. 1979; 142: 30-43.
