Abstract
One of the many bone changes that occur with aging is “cortical drift”, the absorption and deposition of bone on the endosteal and periosteal side, respectively, which results in bone enlargement in some but not all metaphyses. The distal tibia is one of the most fractured sites in the body and where anatomically shaped implants are mostly used. The economic viability of these implants depends on the maintenance of bone contour throughout life. MRI sagittal ankle images from 422 patients aged 18 to 100 years were analyzed and total distal tibia diameter measured. No correlation was observed between the parameters age and distal tibia diameter (Pearson-0.099), or when individuals were separated by sex (Pearson-0.021 for men and 0.049 for women). When separated by age, patients younger and older than 60 years old had a similar average height (1.65 and 1.62 m, respectively, student’s t- test = 0). This is the first study to evaluate possible age-related distal tibia enlargement. Bone changes with age do not result in distal tibia enlargement and possibly the majority of anatomically shaped bone implants are suitable irrespective of age.
Keywords: Distal tibia; Aging
Introduction
Age-related bone changes age are well described in animal models [1-3], where total bone mass reduction occurs with bone metaphysis, trabecular struts become less thick [2,3], and the cross-sectional moment of inertia increases (bone distribution around the central axis) [2]. “Cortical drift”, the absorption of cortical bone on the endosteal surface and deposition of bone on the periosteal surface, could compensate for the decline in bone mass since it expands the outer diameter [4-6]. The amount of total bone widening at different body sites [7,8] and whether bone proportionality is maintained remains controversial [9]. Non-proportional bone enlargement with aging may alter the bone surface contour between people of different ages [10].
In orthopedics, fracture reduction is essential for successful bone healing [11]. Bone reduction is achieved by connecting the bone surface and the micro relief, and irregularities are used as contact parameters [11,12]. In comminuted fractures, micro bone relief is lost, resulting in poorly aligned postoperative cases [12,13]. This is even more important for the metaphyseal bone, because articular fractures must not have an uneven surface [13,14]. Perfect reduction in ankle fractures is crucial because of the high load in a small area [13,14].
To solve these cases, many anatomically designed bone implants were created for the distal tibia [15,16]. There are still no data available to show the real effectiveness of these implants [15,16], little information is published about bone surface variance in the population [13,16], and even less is known about these variances between populations of different countries [13,19]. Thus, these implants may not be precise enough to provide the necessary anatomical alignment [16,17].
If bone size changes with aging, the bone surface could differ between young and older patients. As such, implants should be distinguished between these individuals, and it is important to understand if this change differs between sexes [18-20]. To determine distal tibia width and if there is a perceptible age-related size difference, 422 ankle Magnetic Resonance Imaging (MRI) scans were evaluated and the distal tibia diameter measured.
Materials and Methods
After institutional ethical committee approval, 422 ankle MRIs (one ankle per patient) from the archives of the radiology department, taken between 2017 and 2019, were retrospectively analyzed. Inclusion criteria were sagittal ankle MRIs, age between 18 and 100 years old and no ankle bone abnormalities after careful analysis by the radiologist and orthopedic surgeon. Measurements were taken using Carestream’s Vue Motion Viewer®.
The decision to use MRI images was based on the fact that there were more MRIs available at the department than ankle CT scans, which could be another option. MRI correlation between images and in vivo findings are well described [21,22]. Ankle x-rays were not used because of the less reliable correlation resulting from the variations in the distance between the ankle and x-ray ampoule [23]. Distal tibia diameter was determined in the sagittal MRI view, where the largest measurement was obtained using the oblique view of former cartilage growth scar tissue (Figure 1).
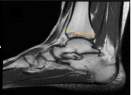
Figure 1: Distal tibia width assessment at sagittal cut T1; the largest measure
obtained in a straight line at the former cartilage growth.
Intra and interobserver reliability were assessed by applying the Intraclass Correlation Coefficient (ICC). Thirty-seven ankles were used in this analysis. Correlations of 0.81 to 0.99 were considered near perfect; 0.61 to 0.80, substantial; 0.41 to 0.60, moderate, 0.21 to 0.40, fair; and slight if less than or equal to 0.20 [21]. Age-related distal tibia enlargement was determined using Pearson’s coefficient between age and distal tibia width and by comparing patients under and over 60 years old (Figure 1).
Results
Average distal tibia diameter of the population was 38.5 mm (SD 38.12-38.87) and no correlation with age was observed (Pearson-0.099) (Figure 2). Distal tibia diameter did not change with age when the analyses were separated by sex (Pearson-0.021 for men and 0.049 for women) (Table 1) (Figure 2). When divided by age, patients under and over 60 years old had similar average height (1.65 and 1.62 respectively, student’s t-test=0.012) and distal tibia diameter (38.4 and 37.7 mm respectively, -student’s t-test=0.42).
Average Age
43 years
SD (41.46-44.53)
Sex distribution
56.45% female
Average Height
1.65 m
SD (1.63-1.66)
Distal Tibia Diameter
38.5 mm
SD (38.12-38.87)
Distal Tibia Women
36.5mm
SD (36.19-36.80)
Height Women
1.61 m
SD (1.59-1.62)
Distal Tibia Men
42 mm
SD (41.51-42.48)
Height Men
1.72 m
SD (1.69-1.74)
Table 1: Population characteristics.
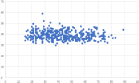
Figure 2: Graphic distribution correlation between distal tibia size (y) and patient age (x).
Discussion
Bone enlargement is thought to be an important compensation for bone mass loss in age-related osteopenia [24]. Few and conflicting literature studies have addressed if, where or how much enlargement actually occurs. Since trabecular bone microstructural properties are heterogeneous throughout the skeleton, evolution, and changes in bone shape with age vary between skeletal sites [6,8,25-29]. Significant differences between men and women are observed at some, but not all sites [29]. Age-dependent rib enlargement was observed [25] in the distal radius [25] and femoral neck [6,8], but not in the vertebral body [2,10,26,27] or ilium [28]. Although understanding the external distal tibia contour behavior with aging is essential since there are many anatomically designed ankle implants used to restore the anatomy contour of this region [15,16], one of the most fractured bones in the body [30], this is the first study to address age-related distal tibia enlargement.
No correlation was observed between distal tibia metaphysis diameter values and age, but some bias could have occurred. The average age (43 years) characterizes a relatively young group, which could affect the results, since fewer patients over 60 years old were included (only 64 of the 422 patients). However, given that Pearson`s coefficient was consistent, and the total number of patients analyzed significant, this fact does not seem relevant.
Another possible bias could be a decrease in distal tibia size in older patients in Brazil, as a different nutritional environment in childhood could result in smaller older adults, with smaller bones. The average height of patients under and over 60 years old was very similar (1.65 and 1.62 m respectively), as was distal tibia diameter (38.4 and 37.7 cm respectively). The absence of distal tibia enlargement cannot be due to consistent intergroup height differences since none were observed. The use of MRI to analyze detailed bone structure could affect the precision of these measures, but the literature has shown good correlation between MRI and anatomical measurements [22].
Anatomically shaped implants designed for the distal tibia can probably be used with no modifications in adults or older patients as effective distal tibia enlargement with aging do not occur. To confirm this hypothesis, studies should address the micro contour details in both groups [16]. Little is known about micro bone contour variations between people in general, and knowing these patterns could result in more precise implants and reduce the high failure rate in restoring anatomical alignment in particular comminuted fractures [31].
Conclusion
Age-related bone metaphysis enlargement is uneven and differs between bone sites. The distal tibia does not widen with age. This finding may help calculate the economic viability of new anatomically shaped implants.
References
- Zhang R, Gong H, Zhu D, Ma R, Fang J, Fan Y. Multi-level femoral morphology and mechanical properties of rats of different ages. Bone. 2015; 76: 76-87.
- Chen H, Zhou X, Washimi Y, Shoumura S. Three-dimensional microstructure of the bone in a hamster model of senile osteoporosis. Bone. 2008; 43: 494- 500.
- Compston JE, Mellish RW, Garrahan NJ. Age-related changes in iliac crest trabecular microanatomic bone structure in man. Bone. 1987; 8: 289-292.
- Black A, Tilmont EM, Handy AM, Scott WW, Shapses SA, lngram DK, et al. A nonhuman primate model of age-related bone loss: a longitudinal study in male and premenopausal female rhesus monkeys. Bone. 2001; 28: 295-302.
- Ferguson VL, Ayers RA, Bateman TA, Simske SJ. Bone development and age-related bone loss in male C57BL/6J mice. Bone. 2003; 33: 387-398.
- Nicks KM, Amin S, Melton LJ 3rd, McCready LK, Riggs BL, Engelke K, et al. Three-dimensional structural analysis of the proximal femur in an agestratified sample of women. Bone. 2013; 55: 179-188.
- Su Y, Wang L, Liu X, Yang M, Yi C, Liu Y, et al. Lack of periosteal apposition in the head and neck of femur after menopause in Chinese women with high risk for hip fractures-A cross-sectional study with QCT. Bone. 2020; 139: 115545.
- Duan Y, Beck TJ, Wang XF, Seeman E. Structural and biomechanical basis of sexual dimorphism in femoral neck fragility has its origins in growth and aging. J Bone Miner Res. 2003; 18: 1766-1774.
- Fyhrie DP, Lang SM, Hoshaw SJ, Schaffler MB, Kuo RF. Human vertebral cancellous bone surface distribution. Bone. 1995; 17: 287-291.
- Junno JA, Paananen M, Karppinen J, Niinimaki J, Niskanen M, Maijanen H, et al. Age-related trends in vertebral dimensions. J Anat. 2015; 226: 434-439.
- Freigang V, Gschrei F, Bhayana H, Schmitz P, Weber J, Kerschbaum M, et al. Risk factor analysis for delayed union after subtrochanteric femur fracture: quality of reduction and valgization are the key to success. BMC Musculoskelet Disord. 2019; 20: 391.
- Bartolotta RJ, Daniels SP, Verret CI, Fufa DT. Current Fixation Options for Elbow, Forearm, Wrist, and Hand Fractures. Semin Musculoskelet Radiol. 2019; 23: 109-125.
- Drosos G, Karnezis IA, Bishay M, Miles AW. Initial rotational stability of distal tibial fractures nailed without proximal locking: the importance of fracture type and degree of cortical contact. Injury. 2001; 32: 137-143.
- Joveniaux P, Ohl X, Harisboure A, Berrichi A, Labatut L, Simon P, et al. Distal tibia fractures: management and complications of 101 cases. Int Orthop. 2010; 34: 583-588.
- Petersik A, Homeier A, Hoare SG, von Oldenburg G, Gottschling H, Schroder M, et al. A numeric approach for anatomic plate design. Injury. 2018; 49: S96-S101.
- Schmutz B, Rathnayaka K, Albrecht T. Anatomical fitting of a plate shape directly derived from a 3D statistical bone model of the tibia. J Clin Orthop Trauma. 2019; 10: S236-S241.
- Goyal KS, Skalak AS, Marcus RE, Vallier HA, Cooperman DR. Analysis of anatomic periarticular tibial plate fit on normal adults. Clin Orthop Relat Res. 2007; 461: 245-257.
- Williams BT, Ahrberg AB, Goldsmith MT, Campbell KJ, Shirley L, Wijdicks CA, et al. Ankle syndesmosis: a qualitative and quantitative anatomic analysis. Am J Sports Med. 2015; 43: 88-97.
- Taylor CE, Henninger HB, Bachus KN. Cortical and medullary morphology of the tibia. Anat Rec (Hoboken). 2020.
- Drew AJ, Tashjian RZ, Henninger HB, Bachus KN. Sex and Laterality Differences in Medullary Humerus Morphology. Anat Rec (Hoboken). 2019; 302: 1709-1717.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33: 159-174.
- Cohen ZA, McCarthy DM, Kwak SD, Legrand P, Fogarasi F, Ciaccio EJ, et al. Knee cartilage topography, thickness, and contact areas from MRI: invitro calibration and in-vivo measurements. Osteoarthritis Cartilage. 1999; 7: 95-109.
- Sposeto RB, Sakaki MH, Godoy-Santos AL, Ortiz RT, Macedo RS, Fernandes TD. Weightbearing Forefoot Axial Radiography-Technical Description and Reproducibility Evaluation. Rev Bras Ortop (Sao Paulo). 2020; 55: 367-373.
- Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003; 349: 327-334.
- Takahashi H, Frost HM. Age and sex related changes in the amount of cortex of normal human ribs. Acta Orthop Scand. 1966; 37: 122-130.
- Scharf HP, Pesch HJ, Lauer G, Seibold H. Form-und Strukturwandel von Wirbelkörpern als Ausdruck ihrer mechanischen Belastung [Changes in form and structure of vertebrae as a manifestation of mechanical loading (author’s transl)]. Microsc Acta Suppl. 1980; 129-134.
- Mosekilde L, Mosekilde L. Sex differences in age-related changes in vertebral body size, density and biomechanical competence in normal individuals. Bone. 1990; 11: 67-73.
- Rissech C, Malgosa A. Ilium growth study: applicability in sex and age diagnosis. Forensic Sci Int. 2005; 147: 165-174.
- Eckstein F, Matsuura M, Kuhn V, Priemel m, Muller R, Link TM, et al. Sex differences of human trabecular bone microstructure in aging are sitedependent. J Bone Miner Res. 2007; 22: 817-824.
- Elsoe R, Ostgaard SE, Larsen P. Population-based epidemiology of 9767 ankle fractures. Foot Ankle Surg. 2018; 24: 34-39.
- Schmidt W, LiArno S, Khlopas A, Petersik A, Mont MA. Stryker Orthopaedic Modeling and Analytics (SOMA): A Review. Surg Technol Int. 2018; 32: 315- 324.
