Research Article
Austin J Gastroenterol. 2014;1(1): 1002.
Effect of Linaclotide in the Treatment of Irritable Bowel Syndrome and Chronic Constipation: a Meta-Analysis
Bechtold ML1*, Ahmad DS1, Esmadi M1, Hinds AM1, Firwana B1, Choudhary A1, Nguyen DL2 and Matteson-Kome ML1
1Department of Gastroenterology and Hepatology, University of Missouri, USA
2Department of Gastroenterology and Hepatology, University of California, USA
*Corresponding author: :Bechtold ML, Department of Gastroenterology and Hepatology, University of Missouri Health Sciences Center, CE405, DC 043.00, Five Hospital Drive, Columbia, MO 65212, USA
Received: May 20, 2014; Accepted: June 09, 2014; Published: June 11, 2014
Abstract
Background: Irritable Bowel Syndrome with Constipation (IBS-C) and functional constipation are common gastrointestinal disorders with limited treatment options. We performed a meta-analysis to estimate the efficacy of different daily doses of oral linaclotide in the management of IBS-C and chronic constipation.
Methods: A search was performed in May 2014. A meta-analysis was performed on randomized controlled trials comparing linaclotide versus placebo to assess the primary outcome (three or more Complete Spontaneous Bowel Movement (CSBM) per week and an increase of at least one CSBM per week from baseline) and secondary outcome (frequency of adverse events). Subgroup analysis was performed by dividing the studies into a high-dose group (290-300 mcg) and low-dose group (145-150 mcg).
Results: Six studies (N=3,654) were included. Linaclotide demonstrated a statistically significant improvement as compared to placebo (OR 3.42; 95% CI: 2.06-5.68; p<0.01) for CSBM. However, linaclotide showed a statistically significant increase in adverse events as compared to placebo (OR 1.28; 95% CI: 1.12-1.48; p<0.01). In subgroup analysis, linaclotide of 145-150 mcg and 290-300 mcg demonstrated statistically significant improvements in CSBM (OR 3.81; 95% CI: 2.55-5.70; p<0.01 and OR 3.84; 95% CI: 2.20-6.69; p<0.01, respectively) as compared to placebo. However, linaclotide of 145-150 mcg and 290-300 mcg revealed a statistically significant increase in adverse events (OR 1.39; 95% CI: 1.09-1.76; p<0.01 and OR 1.24; 95% CI: 1.07-1.45; p<0.01, respectively) as compared to placebo.
Conclusion: Linaclotide appears to be effective in the treatment of IBS-C and chronic constipation but has more adverse events.
Keywords: Linaclotide; Irritable Bowel Syndrome; Constipation; Meta- Analysis
Abbreviations
IBS-C: Irritable Bowel Syndrome with Constipation; CSBM: Complete Spontaneous Bowel Movement; IBS: Irritable Bowel Syndrome; cGMP: Guanylate Cyclase C; DARE: Database of Abstracts of Reviews of Effects; DDW: Digestive Disease Week; ACG: American College of Gastroenterology.
Introduction
Irritable Bowel Syndrome (IBS) is a gastrointestinal syndrome characterized by chronic abdominal pain and altered bowel habits without any other identifiable source. The prevalence of IBS in North America estimated approximately 10-15 percent [1-3], predominately in young patients and women [4]. However, IBS can affect both genders at any age.
The symptoms of IBS adversely affect patient’s health-related quality of life [5]. Furthermore, a significant financial burden on society exists with IBS because of reduced work productivity and an over 50% increase in the use of health-related resources [6]. IBS also accounts for 25-50 percent of all referrals to gastroenterologists [7].
IBS is sub classified according to the predominant alteration in stool form: IBS with constipation (IBS-C), IBS with diarrhea, mixed IBS, and UN sub typed IBS [8]. IBS-C patients, accounting for up to one-third of IBS patients, complain of various symptoms including abdominal pain or discomfort, reduced stool frequency, bloating, hard stools, sensation of incomplete evacuation, and straining [9,10]. Medications targeted to treat IBS-C are often associated with patient dissatisfaction [11]. Lubiprostone is a recent medication that has been shown to improve global symptoms of IBS-C [12]. In September 2012, the FDA approved linaclotide for treatment of IBS-C. Linaclotide, a minimally absorbed peptide, binds to the intestinal epithelium, activating cystic fibrosis transmembrane conductance regulator through guanylate cyclase C (cGMP), and resulting in secretion of chloride and bicarbonate into the intestinal lumen [13,14]. Subsequently, increased luminal fluid secretion and an acceleration of intestinal transit occur [14]. In animal models, accelerated gastrointestinal transit and reduced visceral nociception were noticed with linaclotide treatment [14,15]. cGMP also reduced the firing of afferent pain fibers when applied to the colonic mucosa isolated from mice with visceral hypersensitivity [16]. In humans, linaclotide accelerated colonic transit and improved abdominal pain and constipation associated with IBS-C [17-19].The aim of this study is to perform a meta-analysis of existing clinical trials to estimate the efficacy and safety of different daily doses of oral linaclotide in the management of IBS-C and chronic constipation.
Methods and Materials
Literature search
We searched the electronic literature from MEDLINE/Pub Med, Scopus, CINAHL, Meta Register of Controlled Trials, Database of Abstracts of Reviews of Effects (DARE), and Cochrane databases in May 2014 using the key terms linaclotide, irritable bowel syndrome, and constipation. Moreover, we conducted manual searches of reference lists from relevant papers to identify any additional articles. Additionally, all abstracts from the Digestive Disease Week (DDW) and American College of Gastroenterology (ACG) national meetings were reviewed for potentially relevant abstracts from 2003-2014. We searched all relevant articles irrespective of results or document type. We conducted reference searches in Scopus for all selected articles to identify any newer citing articles. Trials in non-English language and trials in animals were excluded.
Study design
Three investigators (DA, ME and MLB) independently reviewed the titles and abstracts of all citations identified by literature search. All randomized placebo-controlled clinical trials of linaclotide in treatment of IBS-C and chronic constipation was selected. The inclusion criteria were: (1) Studies that examine the effect of linaclotide on symptoms of IBS-C and chronic constipation, (2) studies that were prospective, randomized and placebo controlled published in peer-reviewed journals, and (3) studies in humans. Review articles, retrospective analyses and case reports were excluded.
Data extraction
Two of the authors (DA, ME) extracted data from eligible studies independently using a common data extraction form with any disagreements in the data resolved by a third party (MLB). Articles were selected if they met the inclusion criteria mentioned above.
Assessment of outcomes
The primary outcome assessed was the efficacy of linaclotide therapy in IBS-C and chronic constipation in regards to the effect on the mean number of stools per week while on treatment. The secondary outcome included the frequency of adverse events. Subgroup analysis was also performed by dividing the studies into a high-dose group (290-300 mcg daily) and low-dose group (145-150 mcg daily).
Assessment of study quality
The quality of the studies was assessed using the Jadad scale [20]. Briefly, this scoring scale evaluates each trial according to the quality of the scientific description of the randomization method. The quality scale ranges from 0 to 5 points. Studies with a score of 2 or less were considered as poor quality studies and the ones with a score of 3 or higher were considered high quality studies [20].
Statistical analysis
A meta-analysis was performed comparing linaclotide to placebo for treatment of IBS-C and chronic constipation by calculating pooled estimates of the primary outcome of mean number of stools per week in high- and low-dose groups, and secondary outcome of frequency of adverse events using Odds Ratio (OR) with Mantel-Haenszel (fixed effect) and DerSimonian and Laird (random effects) models. Random effects model was used if statistically significant heterogeneity was noted. Statistical significance was observed if p < 0.05 or range in the confidence interval did not include 1. Publication bias was assessed by funnel plots. Heterogeneity among studies was assessed by calculating the I2 measure of inconsistency, which was considered significant if P < 0.10 or I2 > 50%. %. If heterogeneity was statistically significant, a sensitivity analysis was performed to examine for heterogeneity when certain studies were excluded from the analysis. RevMan 5.2 (Review Manager [Computer program]. Version5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) was utilized for statistical analysis. Publication bias was assessed by funnel plots.
Results
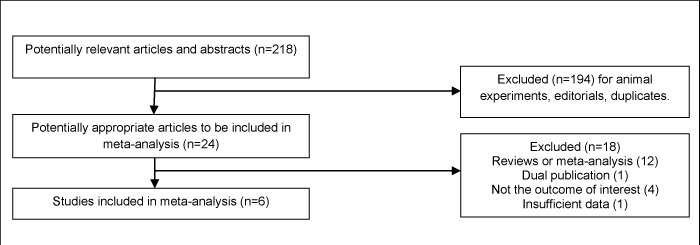
Our initial search identified 218 articles and abstracts. Of the 218 citations identified, we excluded 194 after screening the titles and abstracts, leaving 24 articles for full-text review. Of the 24 articles selected for full review, eighteen were excluded for being systematic review articles, dual publications [21], or not having the outcome of interest. Six articles (n=3,654) ultimately met the inclusion criteria and were included in the meta-analysis [18,22-26] (Figure 1).
Figure 1: Identification of eligible randomized controlled trials.
The six RCTs included in the meta-analysis were performed in the United States with two RCTs including patients from Canada as well [24,25]. They were published between 2009 and 2012 in English. Of the six studies, five investigated the primary outcome of having three or more Complete Spontaneous Bowel Movement (CSBM) per week and an increase of at least one CSBM per week from baseline [18,23-26], and all studies investigated the secondary outcome of frequency of adverse events. The adverse events that were identified are as follows: Diarrhea, allergic reaction, abdominal pain, abdominal distension, dyspepsia, flatulence, nausea, vomiting, proctalgia, urinary tract infection, nasopharyngitis, sinusitis, headache, and upper respiratory infection. Of the adverse effects, diarrhea was the most common. All of the studies were included in high-dose subgroup analysis, while three trials were included in the low-dose subgroup analysis [18,23,24]. Basic characteristics of the included studies are shown in Table 1. All studies were of excellent quality based upon Jadad scores of five. Details of quality assessment are demonstrated in Table 2.
Author and year
Location
Type
No. Patients
Sex (M/F)
Mean age
Duration
Linaclotide dose
Jadad Score
[22]
U.S.
RCT
42
5/37
41.6-48.2
2 weeks
linaclotide 100 mcg daily
linaclotide 300 mcg mg daily
linaclotide 1000 mcg daily
[18]
linaclotide 75 mcg daily
U.S.
RCT
419
33/386
44.4
12 weeks
linaclotide 150 mcg daily
linaclotide 300 mcg daily
linaclotide 600 mcg daily
[23]
linaclotide 75 mcg daily
U.S.
RCT
307
25/282
47.3
4 weeks
linaclotide 150 mcg daily
linaclotide 300 mcg daily
linaclotide 600 mcg daily
[24]
U.S.
RCT
1272
141/1131
47-49
12 weeks
Linaclotide 145 mcg daily
Canada
Linaclotide 290 mcg daily
[25]
U.S.
RCT
800
76/724
43.3-43.7
12 weeks
Linaclotide 290 mcg daily
Canada
[26]
U.S.
RCT
804
84/720
44.0-44.6
26 weeks
Linaclotide 290 mcg daily
Table 1: Characteristics of studies included in the meta-analysis. RCT, randomized controlled trials.
STUDY
STUDY DESIGN
METHOD OF RANDOMIZATION
DOUBLE-BLIND
METHOD OF DOUBLE-BLINDING
DESCRIPTION OF WITHDRAWALS
TOTAL SCORE*
[22]
1
1
1
1
1
5
[18]
1
1
1
1
1
5
[23]
1
1
1
1
1
5
[24]
1
1
1
1
1
5
[25]
1
1
1
1
1
5
[26]
1
1
1
1
1
5
Table 2: Quality assessment of studies included in this meta-analysis using the Jadad score.
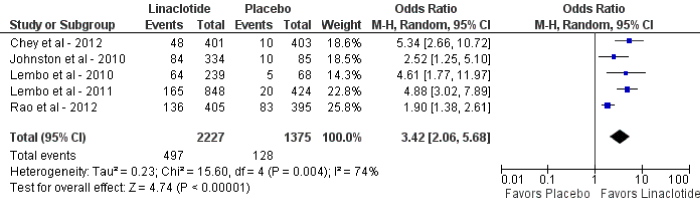
Figure 2: Forest plot demonstrating overall CSBMs for linaclotide compared to placebo.
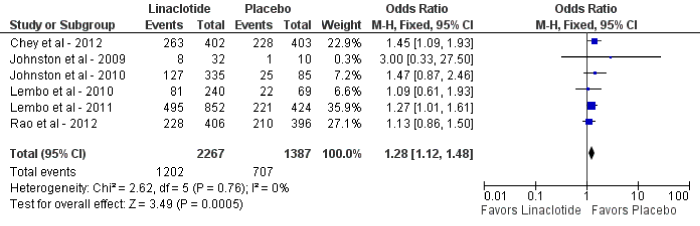
For the primary outcome of CSBM, linaclotide demonstrated a statistically significant improvement as compared to placebo (OR 3.42; 95% CI: 2.06-5.68; p<0.01). The mean percentage of patients who reached the primary endpoint was 9% in the placebo group (128 of 1,375) and 22% in the linaclotide group (497 of 2,227) (figure 2). Statistically significant heterogeneity was observed (I2=74%, p<0.01). Upon sensitivity analysis when one study was removed [25], no heterogeneity was observed with similar results (OR 4.30; 95% CI: 3.11-5.94, p<0.01; I2=0%, p=0.41). The pooled number of patients’ needed-to-treat (NNT) with linaclotide to reach the primary end point was 8. For the secondary outcome, linaclotide showed a statistically significant increase in adverse events as compared to placebo (OR 1.28; 95% CI: 1.12-1.48; p<0.01) (figure 3).
Figure 3: Forest plot demonstrating overall adverse effects for linaclotide compared to placebo.
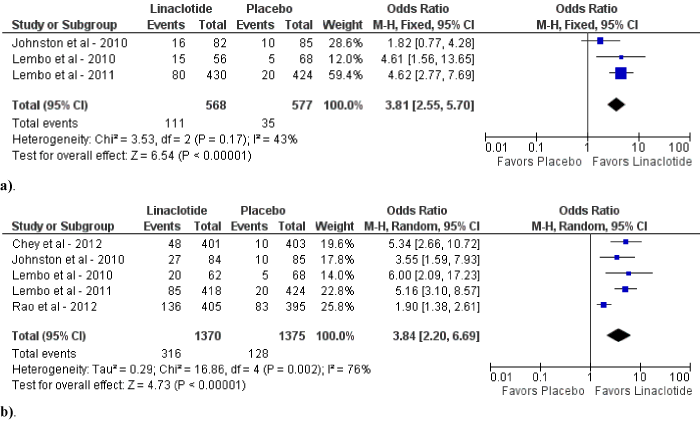
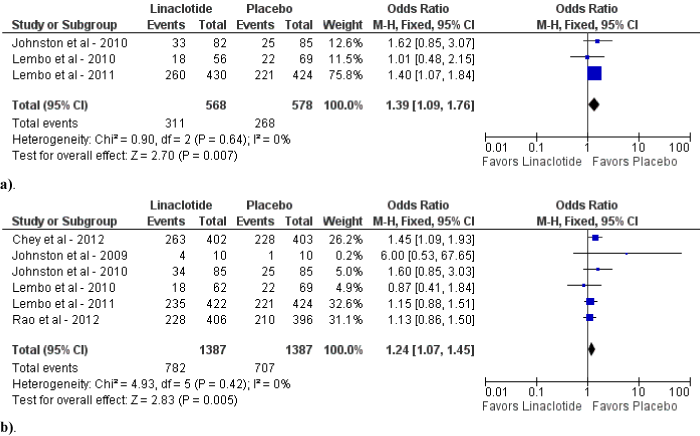
Figure 4: Forest plot demonstrating CSBMs for linaclotide compared to placebo at 145-150 mcg daily (a) and 290-300 mcg daily (b).
Upon subgroup analysis, linaclotide of 145-150 mcg and 290- 300 mcg daily demonstrated statistically significant improvements in CSBM (OR 3.81; 95% CI: 2.55-5.70; p<0.01; I2=43%, p=0.17 and OR 3.84; 95% CI: 2.20-6.69; p<0.01; I2=76%, p<0.01, respectively) as compared to placebo (figure 4a and b). Sensitivity analysis for 290-300 mcg daily dose for CSBM revealed similar findings after elimination of one study [25] (OR 4.93; 95% CI: 3.49-6.97; p<0.01; I2=0%, p=0.84). However, linaclotide of 145-150 mcg and 290-300 mcg daily revealed a statistically significant increase in adverse events (OR 1.39; 95% CI: 1.09-1.76; p<0.01 and OR 1.24; 95% CI: 1.07-1.45; p<0.01, respectively) as compared to placebo. (figure 5a and b).
Figure 5: Forest plot demonstrating adverse effects for linaclotide compared to placebo at 145-150 mcg daily (a) and 290-300 mcg daily (b).
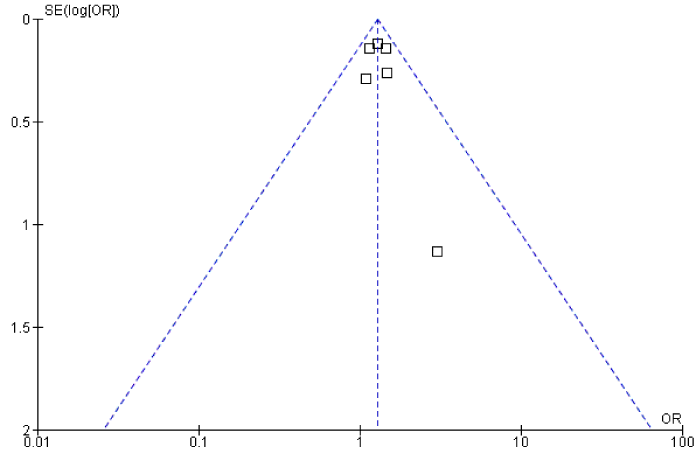
No publication bias was noted for all outcomes (figure 6).
Figure 6: Funnel plot showing no publication bias.
Discussion
As the diagnoses of irritable bowel syndrome and chronic constipation rises, we continue to have difficulty with effective treatment and limited treatment options. Despite two previous medications being approved by the FDA for treatment of IBS-C, tegaserod and lubiprostone [12], tegaserod was removed from the market in 2007 for significant cardiovascular events [12,24], leaving patients with only one approved treatment option. In September 2012, the FDA approved linaclotide for treatment of IBS-C, essentially doubling our treatment options. Over the past six years, six studies have evaluated the use of linaclotide in this population which utilized varying doses and demonstrated varied results [18,22-26].
Our meta-analysis of these six studies demonstrated that linactolide had a statistically significant improvement in the primary outcome as compared to placebo. It also established that there was a statistically significant difference in adverse outcomes with linactolide over placebo. Both current dosing strategies of linaclotide 145-150 mcg and 290-300 mcg daily had improvements in primary outcomes as well as increased adverse effects. Although there is an increase incidence of adverse effects with treatment of linactolide, regardless of dosage, it does seem to provide benefit for patients with IBS-C, especially in the face of limited options.
The strengths of our meta-analysis are numerous. A comprehensive search was performed, allowing for the maximum number of relevant studies to be involved in the meta-analysis. All studies used in the meta-analysis were randomized controlled trials maximizing the relevancy of the studies. Five out of the six studies reached the primary endpoint, maximizing the significance of the study. Also, no publication bias noted. However, some limitations of this meta-analysis are present. Most studies did not include other commonly used treatments for IBS-C, only placebo, so comparisons to other current options are not available such as lubiprostone, currently the only other FDA-approved medication. Of the six studies done, a total of 31% of patients reached the primary end-point, leading to a smaller number of patients maximized in the studies. Third, upon reviewing authors, most studies were conducted by the same group. This may introduce bias given lack of outside trials. However, given the limited trials on this subject, all the RCTs were included in the meta-analysis. Fourth, the RCTs varied in time for which patients were followed, ranging from two weeks to 26 weeks. Fifth, doses in the RCTs varied slightly. To minimize its affect, our subgroup analysis pooled ranges, 145-150 mcg and 290-300 mcg. Finally, in Lembo et al, two parallel trials were presented with similar design and presented in one manuscript. For simplicity, the two trials data were combined into one.
Conclusion
Linactolide use results in significant improvement in complete spontaneous bowel movements per week when compared to placebo. Although there is increase in adverse events with use of linactolide, it seems reasonable to consider it an effective treatment for IBS-C especially in the setting of minimal effective treatments available for patients. In the future there needs to be further research into dosing effectiveness as well as RCT comparing linactolide and lubiprostone.
References
- Hahn BA, Saunders WB, Maier WC. Differences between individuals with self-reported irritable bowel syndrome (IBS) and IBS-like symptoms. Dig Dis Sci. 1997; 42: 2585-2590.
- Drossman DA, Li Z, Andruzzi E, Temple RD, Talley NJ, Thompson WG, et al. householder survey of functional gastrointestinal disorders. Prevalence, sociodemography, and health impact. Dig Dis Sci. 1993; 38: 1569-1580.
- Talley NJ, Zinsmeister AR, Van Dyke C, Melton LJ 3rd. Epidemiology of colonic symptoms and the irritable bowel syndrome. Gastroenterology. 1991; 101: 927-934.
- Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012; 10: 712-721.
- Lackner JM, Gudleski GD, Thakur ER, Stewart TJ, Iacobucci GJ, Spiegel BM. The impact of physical complaints, social environment, and psychological functioning on IBS patients' health perceptions: looking beyond GI symptom severity. Am J Gastroenterol. 2014; 109: 224-233.
- Paré P, Gray J, Lam S, Balshaw R, Khorasheh S, Barbeau M, et al. Health-related quality of life, work productivity, and health care resource utilization of subjects with irritable bowel syndrome: baseline results from LOGIC (Longitudinal Outcomes Study of Gastrointestinal Symptoms in Canada),a naturalistic study. Clin Ther 2006; 28: 1726–1735.
- Everhart JE, Renault PF. Irritable bowel syndrome in office-based practice in the United States. Gastroenterology. 1991; 100: 998-1005.
- Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology 2006; 130: 1480 -1491.
- Drossman DA, Camilleri M, Mayer EA, Whitehead WE. AGA technical review on irritable bowel syndrome. Gastroenterology 2002; 123: 2108 –2131.
- Mayer EA. Clinical practice: irritable bowel syndrome. N Engl J Med 2008; 358: 1692 –1699.
- Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm 2004; 10: 299 –309.
- Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, et al. American College of Gastroenterology Task Force on Irritable Bowel Syndrome. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009; 104: S1–S35.
- Hannig G, Tchernychev B, Kurtz CB, Bryant AP, Currie MG, Silos-Santiago I. Guanylate cyclase-C/cGMP: an emerging pathway in the regulation of visceral pain. Front Mol Neurosci. 2014; 7: 31.
- Bryant AP, Busby RW, Bartolini WP, Cordero EA, Hannig G, Kessler MM, et, al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci2010; 8: 19–20.
- Eutamene H, Bradesi S, Larauche M, Theodorou V, Beaufrand C, Ohning G, et al. Guanylate cyclase C-mediated antinociceptive eff ects of linaclotide in rodent models of visceral pain . Neurogastroenterol Motil 2010; 22: 312–322.
- Castro J, Martin C, Hughes P, Silos-Santiago A, Kurtz C, Blackshaw L, et al. A novel role of cyclic GMP in colonic sensory neurotransmission in healthy and TNBS-treated mice. Gastroenterology 2011; 140: S-538
- Layer P, Stanghellini V. Review article: Linaclotide for the management of irritable bowel syndrome with constipation. Aliment PharmacolTher. 2014; 39: 371-384.
- Johnston JM, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, et al. Linaclotide improves abdominal pain and bowel habits in a phase IIb study of patients with irritable bowel syndrome with constipation. Gastroenterology 2010; 139:1877 –1886.
- Andresen V, Camilleri M, Busciglio IA, Grudell A, Burton D, McKinzie S, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome . Gastroenterology 2007; 133: 761–768.
- Jadad AR, McQuay HJ. A high-yield strategy to identify randomized controlled trials for systematic reviews. Online J Curr Clin Trials. 1993: 33.
- Quigley EM, Tack J, Chey WD, Rao SS, Fortea J, Falques M, et al. Randomised clinical trials: linaclotide phase 3 studies in IBS-C - a prespecified further analysis based on European Medicines Agency-specified endpoints. Aliment Pharmacol Ther. 2013; 37: 49-61.
- Johnston JM, Kurtz CB, Drossman DA, Lembo AJ, Jeglinski BI, MacDougall JE, et al. Pilot study on the effect of linaclotide in patients with chronic constipation. Am J Gastroenterol. 2009; 104: 125-132.
- Lembo AJ, Kurtz CB, Macdougall JE, Lavins BJ, Currie MG, Fitch DA, et al. Efficacy of linaclotide for patients with chronic constipation.Gastroenterology. 2010; 138: 886-895.
- Lembo AJ, Schneier HA, Shiff SJ, Kurtz CB, MacDougall JE, Jia XD, et al. Two randomized trials of linaclotide for chronic constipation. N Engl J Med.2011; 365: 527-536.
- Rao S, Lembo AJ, Shiff SJ, Lavins BJ, Currie MG, Jia XD, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol. 2012 ; 107: 1714-1724.
- Chey WD, Lembo AJ, Lavins BJ, Shiff SJ, Kurtz CB, Currie MG, et al. Linaclotide for irritable bowel syndrome with constipation: a 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol. 2012 ; 107: 1702-1712.