Special Article-Inflammatory Bowel Disease
Austin J Gastroenterol. 2014;1(3): 1014.
A Unique Case Report of Solitary Transverse Colon Perforation in Behcet’s Disease
Jennifer L Bennett1, Caitlin W Hicks1, Linda Lee2 and Michael R Marohn1*
1Department of General Surgery, Johns Hopkins Hospital, USA
2Department of Gastroenterology, Johns Hopkins Hospital, USA
*Corresponding author: :Michael R Marohn, Department of General Surgery, The Johns Hopkins Hospital, 600 N Wolfe Street, Blalock 618, Baltimore, MD 21287, USA
Received: July 31, 2014; Accepted: August 18, 2014; Published: August 20, 2014
Abstract
Behcet’s disease (BD) is a multi-systemic inflammatory vasculitis with a highly variable and relapsing course. We describe a rare and interesting presentation of severe BD involving a focal perforation of the transverse colon, followed by a concise overview of the natural history, pathogenesis, clinical presentation, and treatment of BD. Gastrointestinal (GI) ulceration can occur throughout the small bowel and colon, although solitary lesions are often found in the terminal ileum whereas in the colon multiple shallower ulcers spread throughout are more typical presentations. Despite our patient’s lesion being in the transverse colon, the remainder of our patient’s bowel was unaffected. First line treatment for gastrointestinal perforation is surgical resection, which our patient promptly underwent. This report highlights the importance of maintaining a high index of suspicion for potential life-threatening complications among BD patients presenting with GI symptoms such as nausea, vomiting, diarrhea and abdominal pain. It also highlights the lack of current guidelines pertaining to post-operative surveillance colonoscopy and medical management in this population.
Keywords: Behcet’s disease; Colon perforation; Bowel resection; Colonoscopy; Screening
Introduction
Behcet’s disease (BD) is a multi-systemic inflammatory vasculitis with a highly variable and relapsing course [1]. It characteristically presents with recurrent painful oral aphthae (apthous ulcers), but can also have a number of different systemic manifestations including ocular, urogenital and cutaneous lesions, neurologic and vascular disease, arthritis, and gastrointestinal involvement. No pathognomonic diagnostic test is currently available, so BD is an exclusively clinical diagnosis [2]. The recently developed International Criteria for BD, which uses a point scale system based on multination data from 27 countries, can be used to guide the diagnosis and classification of BD [2].However, physician pattern recognition of the key disease manifestations, along with a high index of suspicion in certain populations, is essential for early detection and management.
BD may involve the gastrointestinal (GI) tract in up to 10- 50% of patients [3-5]. The majority of patients (75-88%) with GI manifestations have terminal ileum and cecal involvement that is typically characterized by a single, deep localized lesion [3,6]. Colonic involvement is less common, and usually presents as multiple shallower lesions spread diffusely throughout the colon [4,7]. GI perforation is rare, occurring in less than 1% of cases [8], but in this group recurrence rates are high [8]. We describe a unique patient with BD who presented with a single perforation of his transverse colon, to emphasize the need for surgeon familiarity with this disorder because when it does occur, it has important implications due to high recurrence rate and therefore requires close follow-up.
Case Presentation
MA is a 32-year-old male from the United Arab Emirates who was first diagnosed with BD at the age of 22 after a 4-year history of recurrent oral aphthous ulcers that had gradually progressed to include episcleritis, scrotal ulcerations, erythema nodosum, and chronic fatigue and muscle aches. He had no family history of BD or other autoimmune disease, and was otherwise healthy. Following his diagnosis, MA was treated initially with prednisone and etanercept. These were tapered over the course of a year to a maintenance regimen involving only colchicine, with occasional short courses of prednisone for intermittent disease flares, that controlled his symptoms relatively well for.
Ten years after he was diagnosed with BD, MA began experiencing increasing fatigue, sleeplessness, and abdominal discomfort. Three months later, he experienced an episode of intense abdominal pain and fevers to 38.4C that prompted admission to the hospital. He was treated initially with steroids and antibiotics for a presumed BD flare. However, computer tomography (CT) imaging revealed a colonic perforation, and he was taken urgently to the operating room for an exploratory laparotomy. Inspection of the bowel demonstrated a frank perforation with a single solitary lesion in the transverse colon that was managed with resection and formation of an end colostomy.
One month following surgery, colonoscopy showed normal terminal ileum and colonic stump; with a residual ulcer in the left colon at 90 cm. MA was maintained on prednisolone 20 mg daily with good symptomatic control. Interval surveillance colonoscopy two months later showed near-resolution of disease with no ulceration. He was started on azathioprine and returned to the operating for a laparoscopic-assisted colostomyreversal, performed without incident. The patient’s post-operative course was largely unremarkable with the exception of delayed wound healing treated with twice-daily wound packing.
At his one-month follow-up visit, MA showed no evidence of residual BD symptoms with the exception of a single small solitary oral aphthous ulcer. He was tapered off steroids, but advised to maintain azathioprine treatment long-term given the severity of his disease. He was also advised to undergo a colonoscopy 6-months post-operatively, and every 1-3 years thereafter to monitor for his increased risk of recurrent GI ulceration. He has since returned to his home in the United Arab Emirates, and is reportedly doing well.
Discussion
BD is a rare multi-systemic vasculitic disorder; its prevalence varies geographically from 80-370 patients per 100,000 inhabitants in Turkey to 13.5-20 per 100,000 in Asia and the Middle East to 1-2 per 1 million in the United States [1,9]. It has a higher prevalence along the historic “silk route,” including Middle and East Asia [5,10,11]. Disease severity tends to be worse in patients from these countries, as well as in male patients and patients with a young age of disease onset [12,13]. In the case that we present, MA fit the epidemiologic profile of severe BD very well. He is male, first developed symptoms of BD at age 18, and originates from the Middle East. However, MA’s case was unique in that he presented with a single perforated ulcer of the transverse colon that required emergent surgical management. Isolated colon perforations are rare in BD patients, with few reports of isolated sigmoid or transverse colon perforations [7,14].
Gastrointestinal involvement is noted in 10-50% of BD cases [3-5], but GI ulceration is rare and usually presents as a single, deep localized lesion in the terminal ileum, or as multiple shallower lesions spread diffusely through the colon [4,7,15]. Of note, MA’s terminal ileum was unaffected by disease, and despite close inspection of the bowel there was no intra-operative evidence of the multiple ulcerations usually found in patients with distal GI involvement. BD patients who are affected by GI lesions generally have a poor prognosis with 5-10% requiring surgery for perforation, hemorrhage, failed medical therapy, obstruction, ileus, or peritonitis at some point in the course of their disease [4,10]. Operative resection is first-line therapy for ulcerative perforation, although controversy exists regarding the appropriate length of bowel to resect in these cases [16]. Recommendations range from removing as much as 60 cm, including the terminal ileum, to a more conservative approach that involves removing only the grossly involved bowel [7]. Given the propensity for BD-related GI ulceration to present as a solitary lesion in the terminal ileum versus multifocal disease in the distal colon, the appropriate resection length should probably be based on the location of disease and the intra-operative findings. In the case of MA, there was no evidence of GI perforation aside from the solitary lesion identified in his transverse colon, and thus a relatively short segment of bowel was removed. Certainly there was a risk of missing one or more non-perforating lesions that were not visible extraluminally, and the perforation location in the transverse colon also raised concern about his potential to have multifocal disease. In this case, intra-operative endoscopy may have been helpful to screen for additional ulceration and to guide the appropriate length of bowel to be resected.
BD patients whom require surgery are at higher risk for post-operative morbidity [16]; with complications related to wound infection or dehiscence, sepsis, hematemesis, and/or melena estimated to occur in 36-44% of cases [8,17]. Delayed wound healing is often observed, due in part from to the cutaneous hyper-reactivity response that occurs even after minor skin trauma in Behcet’s [4,13]. The ubiquitous use of immunosuppressive agents in this population likely also plays a role; steroids in particular have been implicated in poor wound healing both in vitro and in vivo [18,19]. As a result, BD patients require close post-operative monitoring, both in the hospital setting and following hospital discharge. MA’s post-operative course was largely uneventful. The small wound healing issues that he experienced were predictable given his recent high-dose steroid use, and he progressed through the remainder of his stay without further complications.
Among BD patients who have undergone surgical resection of their GI disease, as many as 65% will experience disease recurrence within 6 months of their initial operation and the risk of recurrence increases to as high as 87% at two years follow-up [8,10,20]. Recurrence often occurs near the site of the original bowel anastomosis, and may be the reason for reoperation in up to 44% of cases [3,8,10,20]. The use of intra-operative endoscopy to guide resection margins has been shown to reduce rates of disease recurrence, likely due to the fact that intraluminal imaging allows for the evaluation of the colon for non-perforating ulcerations [21].
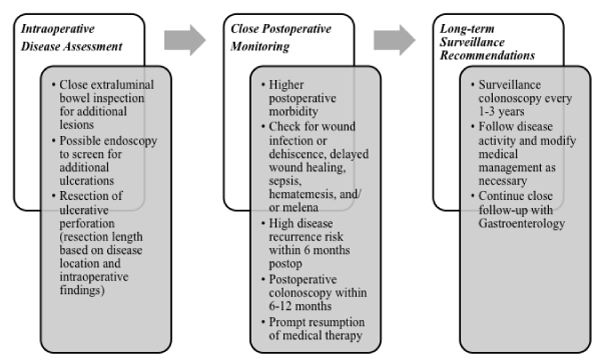
Based on the higher risk of recurrence following surgical resection of GI ulceration in BD, close post-operative surveillance is advised [20,21]. Currently, colonoscopy is the best means to follow BD-related intestinal disease [14]. Although no formal guidelines on the topic exist, we recommend monitoring post-operative BD patients in a similar manner to the post-operative surveillance schema used for patients with inflammatory bowel disease [22]. This recommendation is based on the premise that inflammatory bowel disease and BD may belong to the spectrum of inflammatory disease, making similar follow-up guidelines appropriate until such time that BD-specific recommendations are available [23,24]. Pre-operative evaluation with colonoscopy should be performed in all patients prior to ostomy reversal to ensure disease remission before restoring gastrointestinal continuity [14]. An initial colonoscopy should be performed within 6-12 months of definitive surgery, given the high likelihood of disease recurrence within that timeframe [8,10]. As recommended to MA, we would also advise long-term surveillance with colonoscopies every 1-3 years thereafter, or as clinically indicated, to monitor for ongoing GI disease [ 22], as outlined in Figure 1.
Figure 1: Intra-operative, post-operative, and surveillance screening recommendations for Behcet’s disease. It should be noted that the case we present is a single example of an uncommon disease presenting in rare fashion, so the conclusions that can be drawn are limited. Further studies are needed to determine optimal post-operative care following BD-related perforation.
In addition to long-term disease surveillance with colonoscopy, treatment with medical therapy is also often indicated to keep disease activity low [14]. Utilizing a multidisciplinary approach with BD patients may be necessary to coordinate their complex medical and surgical care [15]. As recommended by inflammatory bowel disease guidelines, early resumption of medical therapy to preclude the likely progression towards disease recurrence is rational [22]. There is currently minimal evidence regarding the optimal post-operative medical therapy for intestinal BD [25], but the use of immunosuppressive agents in conjunction with glucocorticoids have been found to reduce post-operative complications in a few small studies[3,26,27]. Accordingly, in the immediate post-operative period MA was continued on prednisolone and started on azathioprine. Data suggests azathioprine may be beneficial in reducing occurrence of eye involvement, genital ulcerations and GI involvement [24,28]. He appears to be tolerating this strategy well, although follow-up is admittedly limited. As mentioned above, regular colonoscopy is essential to follow disease activity and modify medical treatment strategies as appropriate [17].
The case of MA describes a rare and interesting presentation of severe BD involving a focal perforation of the transverse colon. This report highlights the importance of maintaining a high index of suspicion for potential life-threatening complications among BD patients presenting with GI symptoms. It also highlights the lack of current guidelines pertaining to surveillance colonoscopy and medical management in this population. It should be noted that the case we present is a single example of an uncommon disease presenting in rare fashion, so the conclusions that can be drawn are limited. Further studies are needed to determine optimal post-operative care following BD-related perforation, but in the interim we recommend utilizing existing inflammatory bowel disease recommendations to maintain close follow-up of disease activity and recurrent ulceration.
References
- Bayraktar Y, Ozaslan E, Van Thiel DH. Gastrointestinal manifestations of Behcet's disease. J Clin Gastroenterol. 2000; 30: 144-154.
- International Team for the Revision of the International Criteria for Behcet's Disease (ITR-ICBD), Davatchi F, Assaad-Khalil S, Calamia KT, Crook JE, Sadeghi-Abdollahi B, et al. The International Criteria for Behcet's Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol. 2013.
- Choi IJ, Kim JS, Cha SD, Jung HC, Park JG, Song IS, et al. Long-term clinical course and prognostic factors in intestinal Behçet's disease. Dis Colon Rectum. 2000; 43: 692-700.
- Dowling CM, Hill AD, Malone C, Sheehan JJ, Tormey S, Sheahan K. Colonic perforation in Behcet's syndrome. World J Gastroenterol. 2008; 14: 6578-6580.
- Ebert EC. Gastrointestinal manifestations of Behçet's disease. Dig Dis Sci. 2009; 54: 201-207.
- Yurdakul S, Tüzüner N, Yurdakul I, Hamuryudan V, Yazici H. Gastrointestinal involvement in Behçet's syndrome: a controlled study. Ann Rheum Dis. 1996; 55: 208-210.
- Turan M, Sen M, Koyuncu A, Aydin C, Arici S. Sigmoid colon perforation as an unusual complication of Behçet's syndrome: report of a case. Surg Today. 2003; 33: 383-386.
- Kasahara Y, Tanaka S, Nishino M, Umemura H, Shiraha S, Kuyama T. Intestinal involvement in Behçet's disease: review of 136 surgical cases in the Japanese literature. Dis Colon Rectum. 1981; 24: 103-106.
- Grigg EL, Kane S, Katz S. Mimicry and deception in inflammatory bowel disease and intestinal behçet disease. Gastroenterol Hepatol (N Y). 2012; 8: 103-112.
- Naganuma M, Iwao Y, Inoue N, Hisamatsu T, Imaeda H, Ishii H, et al. Analysis of clinical course and long-term prognosis of surgical and nonsurgical patients with intestinal Behçet's disease. Am J Gastroenterol. 2000; 95: 2848-2851.
- Saleh Z, Arayssi T. Update on the therapy of Behçet disease. Ther Adv Chronic Dis. 2014; 5: 112-134.
- Zouboulis CC, Kötter I, Djawari D, Kirch W, Kohl PK, Ochsendorf FR, et al. Epidemiological features of Adamantiades-Behçet's disease in Germany and in Europe. Yonsei Med J. 1997; 38: 411-422.
- Mat C, Yurdakul S, Sevim A, Özyazgan Y, Tüzün Y. Behçet's syndrome: facts and controversies. Clin Dermatol. 2013; 31: 352-361.
- Isik B, Ara C, Kirimlioglu H, Sogutlu G, Yilmaz M, Yilmaz S, et al. Single or multiple perforations with varying locations as a complication of intestinal Behçet's disease: report of three cases. Scand J Gastroenterol. 2005; 40: 599-603.
- Dalvi SR, Yildirim R, Yazici Y. Behcet's Syndrome. Drugs. 2012; 72: 2223-2241.
- Moon CM, Cheon JH, Shin JK, Jeon SM, Bok HJ, Lee JH. Prediction of free bowel perforation in patients with intestinal Behçet's disease using clinical and colonoscopic findings. Dig Dis Sci. 2010; 55: 2904-2911.
- Lee KS, Kim SJ, Lee BC, Yoon DS, Lee WJ, Chi HS, et al. Surgical treatment of intestinal Behçet's disease. Yonsei Med J. 1997; 38: 455-460.
- Franz MG, Steed DL, Robson MC. Optimizing healing of the acute wound by minimizing complications. Curr Probl Surg. 2007; 44: 691-763.
- Wagner AE, Huck G, Stiehl DP, Jelkmann W, Hellwig-Bürgel T. Dexamethasone impairs hypoxia-inducible factor-1 function. Biochem Biophys Res Commun. 2008; 372: 336-340.
- Jung YS, Yoon JY, Lee JH, Jeon SM, Hong SP, Kim TI, et al. Prognostic factors and long-term clinical outcomes for surgical patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2011; 17: 1594-1602.
- Iida M, Kobayashi H, Matsumoto T, Okada M, Fuchigami T, Yao T. Postoperative recurrence in patients with intestinal Behçet's disease. Dis Colon Rectum. 1994; 37: 16-21.
- Regueiro M. Management and prevention of postoperative Crohn's disease. Inflamm Bowel Dis. 2009; 15: 1583-1590.
- Ketch LL, Buerk CA, Liechty D. Surgical implications of Behçet's disease. Arch Surg. 1980; 115: 759-760.
- Jung YS, Cheon JH, Hong SP, Kim TI, Kim WH. Clinical outcomes and prognostic factors for thiopurine maintenance therapy in patients with intestinal Behcet's disease. Inflamm Bowel Dis. 2012; 18: 750-757.
- Saenz A, Ausejo M, Shea B, Wells G, Welch V, Tugwell P, et al. Pharmacotherapy for Behcet's syndrome. Cochrane Database Syst Rev. 2000; CD001084.
- Park MC, Hong BK, Kwon HM, Hong YS. Surgical outcomes and risk factors for postoperative complications in patients with Behcet's disease. Clin Rheumatol. 2007; 26: 1475-1480.
- Hatemi G, Yazici Y, Yazici H. Behçet's syndrome. Rheum Dis Clin North Am. 2013; 39: 245-261.
- Hatemi G, Silman A, Bang D, Bodaghi B, Chamberlain AM, Gul A. Management of Behçet disease: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for the management of Behçet disease. Ann Rheum Dis. 2009; 68: 1528-1534.