Case Report
Austin J Gastroenterol. 2014;1(5): 1023.
Colonic Complications from Gastrointestinal Amyloidosis
Reshi Kanuru, Peter P Stanich and Marty M Meyer*
Department of Gastroenterology, Hepatology & Nutrition, The Ohio State University, USA
*Corresponding author: : Marty M Meyer, Department of Gastroenterology, Hepatology,& Nutrition, Wexner Medical Center at The Ohio State University, 395 W 12th Ave, Second Floor, Columbus, OH43210, USA
Received: July 23, 2014; Accepted: September 24, 2014; Published: September 26, 2014
Abstract
Amyloidosis is the deposition of protein fibrils within tissues throughout the body. The accumulation of these proteins leads to multiple organ dysfunction including heart, kidneys, and gastrointestinal tract. In the gastrointestinal system, the accumulation of amyloid can lead to nausea, vomiting, diarrhea, and even uncontrollable bleeding. We present a patient with cirrhosis secondary to hepatic amyloidosis and hematochezia due to diffuse colonic submucosal hemorrhages. Understanding the effects amyloidosis has on the gastrointestinal system will allow us to better identify affected patientsand offer earlier therapy.
Introduction
Amyloidosis is a systemic disease characterized by deposition of protein fibrils in association with a plasma cell dyscrasia or an underlying chronic inflammatory disorder. The gastrointestinal tractis one of the many affected organ systems in amyloidosis. The most common segment affected is the small bowel, but the colon can also be involved. Gastrointestinal complications of amyloidosis include dysmotility, malabsorption, and bleeding. Our case highlights the severe consequences amyloidosis can cause in the gastrointestinal system, specifically those complications that arise in colon.
Case
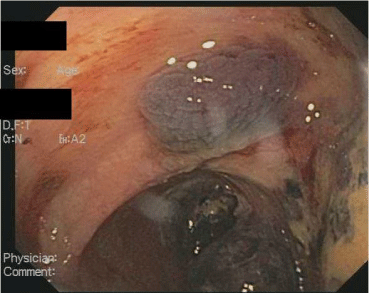
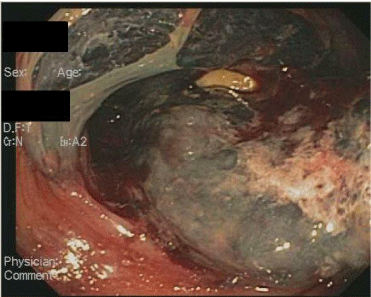
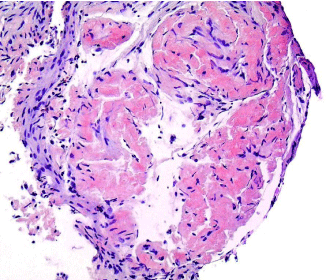
A 58 year old male with newly diagnosed cirrhosis secondary to hepatic amyloidosis presented with hematochezia. Prior to admission he had several months of diarrhea. On admission his hemoglobin was 8.6 g/dL and he had a platelet count of 206,000/uL. He underwent an upper endoscopy significantonly for a widely patent Schatzki’s ring. His colonoscopy showed blood throughout the colon with multiple large submucosal hemorrhages (Figures A&B) starting from the hepatic flexureand continuing into the ascending colon. Diffusely friable mucosa with oozing blood was visualized. No interventions were performed due to the diffuse nature of his lesions. Biopsies of the affected tissue showed amyloid deposits surrounding the colonic vasculatureand scattered in the muscularis mucosa (Figure C). Despite ongoing resuscitation, he had progressive liver dysfunction with ongoing hematochezia and died 10 days after colonoscopy.
Figure A: Submucosal hemorrhage at hepatic flexure.
Figure B: Submucosal hemorrhages in ascending colon.
Figure C: Congo red staining of amyloid in colonic muscularis mucosa under white light.
Discussion
Amyloidosis is categorized into several forms, with the most predominant being AL and AA. AL refers to immunoglobulin light chain deposition commonly associated with plasma cell dyscrasias, such as multiple myeloma and Waldonstrom’s macroglobulinemia. AA amyloidosis is associated with amyloid A, an acute phase reactant, which deposits in tissues in the setting of an inflammatory disorder, such as Crohn’s Disease or rheumatoid arthritis. Both AL and AA can cause severe consequences in the colon due to the accumulation of protein fibrils within tissues. Colonic amyloid deposition can lead to bleeding, ischemia, malabsorption, and pseudo-obstruction.
Amyloid deposition in the colon can cause submucosal hematomas and localized ischemia. The pattern of amyloid deposition plays a large role in the development of these complications. Generally amyloid involves the submucosa with both the interstitium and vasculature wall being common sites of deposition [1]. The deposition of amyloid in the submucosa and submucosal vasculature leads to friability of the vessels resulting in submucosal hematomas. In addition, vascular involvement of AA and AL amyloidosis tends to be circumferential and cause luminal narrowing [2]. The resulting narrowing of colonic vessels can lead to ischemic colitis. Amyloid involvement of blood vessels leads to submucosal hematomas and ischemia with few therapeutic options available. As a result it is important to identify and treat amyloidosis patients early to prevent the morbidity and mortality associated with these complications.
In addition to vascular complications, colonic amyloidosis can result in malabsorption and dysmotility. Diarrhea is one of the predominant presenting symptoms in amyloidosis patients [3]. These symptoms may reflect the prevalence of malabsorption in amyloidosis. Malabsorption likely results from a combination of factors including motility and impaired perfusion of the colon. Amyloid deposits within the submucosa act as a physical barrier to water absorption in the colon. Ongoing malabsorption results in malnutrition. In a recent prospective study looking at 128 newly diagnosed AL amyloidosis patients who were treatment naïve, malnutrition defined by a BMI of less than or equal to 22 and a 10% weight loss was found to be an independent predictor of mortality despite the degree of cardiac involvement [4]. Consequently, nutrition and hydration remains an integral component in the care of amyloid patients. Early identification of malabsorption and dehydration plays a vital role in starting supplemental nutrition and decreasing the mortality risk associated these risk factors.
Amyloidosis can also affect colonic motility, which can lead to intestinal pseudo-obstruction.The colon is composed of an inner circular and outer longitudinal muscle layers that are innervated by the autonomic nervous system, which together are responsible for gastrointestinal motility. The autonomic nervous system triggers motility via the myenteric plexuses. A previous case series examined the location of amyloid deposition in AA and AL amyloidosis to identify any correlation with the development of pseudo-obstruction. Patients with AL amyloidosis had significant deposition of amyloid in the muscularis propria with associated breakdown and loss of function in the myenteric plexuses due to compression [5]. On the other hand, AA amyloidosis has been found to predominately deposit amyloid in the myenteric plexuses leading to nerve cell degeneration [5]. This disparity in distribution reflects the different pathways by which motility is affected in amyloidosis. Though pseudo-obstruction is not specific or sensitive for amyloidosis, the diagnosis of amyloid should be entertained in patients with pseudo-obstruction who have a plasma cell dyscrasia or a chronic inflammatory disorder.
Conclusion
Our case of diffuse colonic submucosal hemorrhages highlights the drastic consequences amyloidosis can have on the gastrointestinal tract. Amyloidosis causes protein deposition in tissues leading to dysmotility, malabsorption, and impaired perfusion. Understanding the presenting symptoms and complications of amyloidosis may help foster early diagnosis in the proper clinical context.
References
- James DG, Zuckerman GR, Sayuk GS, Wang HL, Prakash C. Clinical recognition of AL Type Amyloidosis of the Luminal Gastrointestinal Tract. Clinical Gastroenterology and Hepatology. 2007; 5: 582-588.
- Ohashi K, Takagawa R, Hara M. Mitsuru “Visceral Organ Involvement and Extracellular Matrix Changes in Beta2-Microglobulin amyloidosis - a comparative study with systemic AA and AL Amyloidosis. Virchows Arch. 1997; 430: 479-487.
- Hayman SR, Lacy MQ, Kyle RA, Gertz MA. Primary Systemic Amyloidosis: A Cause of Malabsorption Syndrome. Am J Med. 2001; 11: 535-540.
- Caccialanza R, Palladini G, Klersy C, Cereda E, Bonardi C, Cameletti B. Malnutrition at diagnosis predicts mortality in patients with systemic immunoglobulin light-chain amyloidosis independently of cardiac stage and response to treatment. JPEN J Parenter Enteral Nutr. 2014; 38: 891-894.
- Tada S, Iida M, Yao T, Kitamoto T, Yao T, Fujishima M. Intestinal pseudo-obstruction in patients with amyloidosis: clinicopathologic differences between chemical types of amyloid protein. Gut. 1993; 34: 1412-1417.