
Review Article
Austin J Gastroenterol. 2024; 11(1): 1130.
New Therapeutic Options for Inflammatory Bowel Disease: An Integrative Review
Davi Vieira Ferreira¹*; Ythay Amerson Tavares Saraiva²
1Residente Doctor at Hospital Regional do Cariri, Brazil
2Routine Physician at Hospital Regional do Cariri, Brazil
*Corresponding author: Davi Vieira Ferriera Residente Doctor at Hospital Regional do Cariri, Brazil. Email: davivieiraferreira@gmail.com
Received: January 02, 2024 Accepted: February 07, 2024 Published: February 14, 2024
Abstract
Inflammatory Bowel Diseases (IBD) are chronic inflammatory disorders of the gastrointestinal system that affect thousands of people worldwide. It is an inflammatory condition characterized by changes in mucosal structure, changes in gut microbial composition, and biochemical abnormalities. The main symptoms are systemic inflammatory signs, diarrhea, abdominal pain, rectal bleeding, and weight loss. Despite advances in the treatment of IBD, conventional therapies are still used, which are generally ineffective, as they do not prevent relapses or mucosal healing. Thus, there was a need for new therapeutic means, because a large number of patients undergoing conventional treatment require surgical intervention due to the worsening of the disease later on. However, the new treatment options have been little explored in the literature, with the need for scientific studies on the subject. Thus, this study aims to present new therapeutic options for inflammatory bowel disease and an update on the current status in the clinical development of these new therapeutic classes in IBD. It has been found that new therapeutic options for inflammatory bowel disease offer hope and improved quality of life for patients. As research continues to advance and more effective therapies are developed, the outlook for the treatment of IBD is increasingly positive. However, more clinical trials and investment in research are needed to further improve the available therapeutic options, providing a better life for individuals affected by this debilitating condition.
Keywords: Inflammatory Bowel Diseases; Crohn’s Disease; Ulcerative Colonitis; Management
Introduction
Inflammatory Bowel Disease (IBD) is part of a group of chronic autoimmune diseases. Its etiology is unknown, however, it is characterized by intestinal inflammation and divided into Crohn's Disease (CD), which presents a discontinuous, transmural inflammation affecting the gastrointestinal tract and Ulcerative Colitis (UC), which is restricted to the intestinal mucosa [23,28]. The pathogenesis of CD and UC involves a dysregulated immune response to the commensal microbiota in genetically susceptible individuals [12]. Both disorders are conditions characterized by chronic histological inflammation and impaired quality of life, as CD and UC symptoms include inflammation, diarrhea, abdominal pain, rectal bleeding, and weight loss. The diseases can occur in adolescents and adults, in addition to affecting all sexes [11,25]. CD involves the terminal ileum, cecum, perianal area, and colon, however, it can affect other regions of the intestine and UC affects the rectum and part or all of the colon. The cause of the diseases is still unknown, however, recent studies present evidence that pathogenesis is related to genetic susceptibility, gut microbiota, environmental factors, and immune abnormalities [10]. There are multiple possibilities involved in the pathogenesis of IBD, however, only part of the heritability has been explained by genetic studies [20]. Furthermore, it is worth noting that the growing understanding of the immunopathogenesis of Inflammatory Bowel Disease (IBD) has opened new avenues for the development of more effective therapies than traditional ones. These advances in treatment options targeting different mechanisms of action offer new hope for the proper management of the disease [7]. Cambui and Natali (2015) state that conventional treatments are, for the most part, ineffective, given that they do not prevent recurrent crises, nor the cure of the disease. Thus, there is a need for new therapeutic means, since most patients submitted to conventional treatment require surgical intervention due to the worsening of the disease later. Therefore, there is a need to innovate with the development of new treatments to alter the clinical course of IBD, including fewer clinical relapses, hospitalizations, surgeries, and better quality of life for the patient. Unfortunately, the new treatment options have been little explored in the literature, and there is a need for scientific studies on the subject. Therefore, this research seeks to present an update on the current status in the clinical development of these new therapeutic classes of Inflammatory Bowel Disease.
The general objective of this study is to present the new therapeutic options available for the treatment of inflammatory bowel diseases. The specific objectives are: to present the traditional management that involves the use of sulfasalazine, corticosteroids, antibiotics and immunosuppressants; discuss the therapeutic approach by biological agents of the disease and analyze the benefits of the use of stem cells in the treatment of the disease.
Theoretical Framework
Inflammatory Bowel Diseases (IBDs) are chronic inflammatory disorders of the gastrointestinal system that affect thousands of people worldwide. It is a chronic inflammatory condition characterized by changes in mucosal structure, change in gut microbial composition, and biochemical abnormalities [27.
IBDs have two main clinical forms, Ulcerative Colitis (UC) and Crohn's Disease (CD), which are distinguished by the different clinical manifestations of intestinal inflammation and location. Both diseases are more commonly found in urban areas compared to rural areas and both have different side effects [25].
UC is an inflammation and sores (ulcers) along the lining of the large intestine (colon) and rectum. CD, as far as it is concerned, is characterized by inflammation of the lining of the digestive tract, which can usually involve the deeper layers of the digestive tract, affecting the small and large intestine, as well as, in rare cases, the upper gastrointestinal tract [13].
In addition, CD can affect any part of the gastrointestinal tract. It usually affects the portion of the small intestine before the large intestine/colon. The areas impacted by the disease are manifested through spots that are next to areas of healthy tissue, and can reach through the multiple layers of the walls of the gastrointestinal tract. In UC, the damaged areas are continuous, starting in the rectum and spreading to the colon, being present only in the innermost layer of the colon lining [9].
Several factors are attributed to the prevalence of CD and UC, such as: geographic location, diet, genetics and inadequate immune response. However, the hypothesis most widely regarded by researchers in the scientific community suggests that IBD is the result of an exaggerated immune response, triggered by environmental factors in relation to altered gut microbiota or pathogenic microorganisms in a genetically prone host. Alteration of the gut microbiota in IBD pathology is a possibility; however, it is unclear whether such an alteration is the cause of intestinal inflammation or a consequence of it, and the way in which these bacteria contribute to the pathogenesis of IBD [13].
Regarding genetic factors, Maranhão, Vieira and Campos (2015) complement by stating that 163 gene loci related to IBD were found, in which 110 are associated with both diseases, demonstrating the sharing of common genetic bases and, thus, similar mechanisms in their development, while 30 were associated only with CD and 23 with UC.
The main clinical features of IBDs are: diarrhoea, abdominal pain and, in the case of ulcerative colitis, bleeding. CD is characterized by symptoms of diarrhea, abdominal pain, weight loss, malaise, anorexia, weight loss, and fever. UC has the following specific manifestations: bloody diarrhea, bladder tenesmus, mucus elimination, abdominal cramps, and urgency to evacuate [22].
The diagnosis of IBD is made through the evaluation of the patient's clinical condition, in line with imaging, laboratory and histopathological tests. It should be performed, first, through an interview conducted by the health professional with the patient, in order to verify symptoms, perform a physical examination and family history (SOUSA, 2017).
Likewise, the use of laboratory diagnostic tools is highlighted, such as: complete blood count, C-Reactive Protein (CRP), Erythrocyte Sedimentation Rate (ESR), serum albumin biochemical parameter, iron deficiency screening, as well as a test that seeks to identify microorganisms that may be related to gastrointestinal changes (coproscopy and stool culture), aiming to exclude other diagnostic possibilities (BARROS et al., 2020).
Barros et al. (2020) complement by presenting the importance of ileo-colonoscopic examination and biopsy, which define the severity and extent of the disease. Histological examination reveals the loss of structure of undifferentiated cell mixtures of all cell types and the presence of inflammatory cells in the lamina propria.
Laboratory tests may show anemia, due to the difficulty of assimilation or blood loss, with an increase in the number of white blood cells, per volume of circulating blood; lower than normal blood potassium concentration in cases of severe diarrhea and a gradual increase in Erythrocyte Sedimentation Rate (ESR), in addition to an increase in C-Reactive Protein (CRP), indicating the presence of inflammation or infection [22].
Treatment of IBD varies depending on the type and symptoms. The purpose of IBD treatment is to reduce inflammation, which can lead not only to symptom relief, but also to a reduction in the risk of complications [2]. It usually involves drug therapy or surgery. The improvement of symptoms, prevention of recurrences, induction of remission in patients and healing of fistulas involves a pharmacological approach that is not so simple, in view of the lack of understanding of the nature of the agents responsible for the inflammatory process, the variations in the pharmacokinetics of the drugs and the unique characteristics of each patient [5].
Thus, there are different approaches to treatment, such as: conventional treatment, which involves [5,30]:
• Use of anti-inflammatory drugs Sulfasalazine, Mesalamine. They work by minimizing irritation in the intestines;
• Use of corticosteroids (prednisone). Keeps the immune system under control and manages flare-ups;
• Use of antibiotics, such as ciprofloxacin and metronidazole , as they treat infections and abscesses);
• Use of immunosuppressants (azathioprine, 6-mercaptopurine, methotrexate, and tacrolimus);
Currently, treatment mediated by biological agents stands out. It is a recent therapy, in which it is targeted to neutralize proteins in the body that are causing inflammation. Some are administered through intravenous (IV) infusions and others are intramuscular injections. The most commonly used are: Infliximab, Adalimumab, Golimunab and Certolizumab [5,30].
Methodology
Study Design
This is an exploratory, descriptive study with a qualitative approach. The main purpose of exploratory searches is to clarify concepts and ideas, providing an overview of a given fact. In this way, it makes it possible to broaden the researcher's experience on the problem in question, deepening his study within the limits of a specific reality. Therefore, it has the plan to obtain the desired results, based on contact with a certain population, and can be used to raise possible problem issues [19].
Descriptive research aims to address the particularities of a target audience. The problem of this study is highlighted by identifying, recording and analyzing the characteristics or factors involved with the phenomenon, without interference from the researcher, as they involve standardized data collection techniques (BRUCH&Ecicr;Z, 2018).
Regarding data collection procedures, this is an integrative literature review. For Roman and Friedlander (2018, p. 109), this type of research "is a method that aims to synthesize results obtained in research on a delimited theme or issue, in a systematic and orderly manner, with the aim of contributing to the knowledge of that theme or issue".
Methodological Procedures
The research was carried out through publications in the form of scientific articles on Inflammatory Bowel Diseases (IBD), using scientific works available in the Virtual Health Library (VHL), which is an online information network coordinated by the Latin American Center for Health Sciences Information (BIREME).
The LILACS, SciELO, BDENF and PubMed databases were used, using the DeSC descriptors in Portuguese and English, such as "Inflammatory Bowel Diseases AND Crohn's Disease", "Inflammatory Bowel Diseases and Ulcerative Colitis" and "Inflammatory Bowel Diseases And Therapeutic Adherence".
The selected articles were those that responded to the objectives of the study, published in the last 5 years, available in Portuguese and English, and that were not repeated, were not monographs, dissertations, theses, review articles, news articles, texts in reviews, non-indexed articles, opinions, editorials or manuals.
Data analysis was performed through Thematic Content Analysis, which consists of three stages: pre-analysis, exploration of the material or coding, and treatment of the results obtained/interpretation. During the pre-analysis stage, hypotheses or assumptions were formulated and reformulated, and the corpus was constituted.
In the stage of exploration of the material, categories were found that were organized according to the expressions or words that were significant. From there, inferences and interpretations were proposed, interrelating them with the theoretical framework initially drawn or opening other avenues around new theoretical and interpretative dimensions.
Through the methodology used in this integrative review, 1457 publications were initially identified. After filtering, a total of 35 articles were obtained. An exploratory reading of these articles was carried out, and subsequently, 27 of them were excluded because they did not meet the established criteria or because they were repeated.
Thus, a total of 8 articles were incorporated into this research. The procedure was divided into stages to ensure a better systematization of knowledge on the topic addressed. Initially, the descriptors were searched in the databases, followed by the reading of the abstracts and objectives of the selected articles. Finally, a complete reading of the articles that met the inclusion criteria established for this review was performed.
Data analysis was performed by reading and interpreting the information obtained from the articles.
The selected studies were organized in a table, presenting the profile of the publications: title, authors, year, objective, method, results obtained, database and scientific journal. The academic findings were analyzed in descriptive and interpretative form.
Results
Description of Selected Studies
An analytical reading of the selected articles was carried out, which allowed the subjects to be organized in order of importance and to synthesize the essential ideas to achieve the objective of the research. Graph 1 shows the distribution of articles according to the databases used.
Graph 1 shows that the database with the highest number of articles selected for this study was Scopus (6 articles), followed by PubMed (2 articles). The studies selected according to methodological approach and language are shown in Table 1.
Variables
Number
Percentage
Methodological Approach
Exploratory and qualitative
7
87,50%
Retrospective cross-sectional study
1
12,50%
Language
Portuguese
0
00,00%
English
100
100%
Spanish
0
11,11%
Source: Own Authorship (2023).
Table 1: Organization of manuscripts according to methodological approach and language.
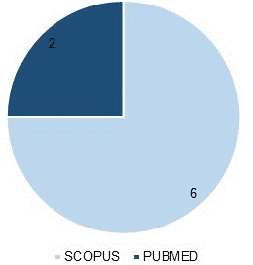
Figure 1: Distribution of articles according to the database used.
Table 2 shows that the methodological approach shows that exploratory and qualitative studies prevailed with 87.50% of the selected articles. Regarding the languages of the publications, there was a predominance of English in 100%.
Author
Title
Goal
Conclusion
Database
Verstockt et al. (2018)
New treatment options for inflammatory bowel diseases
Discuss recent advances in the treatment of IBD, including conventional therapies such as corticosteroids and immunosuppressants, and biologic therapies, which target specific immune system molecules involved in inflammation.
The study presents different treatment options for inflammatory bowel diseases, such as biologic therapies, cytokine inhibitors, monoclonal antibodies, integrin antagonists, and the modulation of the gut microbiome. These approaches offer new hope for the treatment and management of these conditions, improving patients' quality of life
PubMed
Duijvestein et al. (2018)
Novel Therapies and Treatment Strategies for Patients with Inflammatory Bowel Disease
Present current treatment options and strategies and provide an update on the status of programs developing new therapeutic agents for inflammatory bowel disease (IBD)
The results of studies show that biological therapies, such as anti-TNF monoclonal antibodies, have been shown to be effective in fighting intestinal inflammation and remitting the symptoms of inflammatory bowel disease (IBD). Drugs that inhibit inflammatory molecules, such as vedolizumab, and therapies that target specific targets in the immune system, such as JAK inhibitors and IL-12/23, have also shown positive results in controlling inflammation in IBD.
PubMed
Lee, Park and Park (2018)
Novel treatments for inflammatory bowel disease
Discuss new treatments for inflammatory bowel disease
The study by Lee, Park, and Park (2018) provided valuable insight into advances in the treatment of inflammatory bowel disease, highlighting biologic therapies, cytokine inhibitors, leukocyte modulators, novel targeted therapies, and combination approaches as promising options for the management of IBD.
Scopus
Na and Moon (2019)
Perspectives on Current and Novel Treatments for Inflammatory Bowel Disease
Discuss new perspectives on current and new treatments for inflammatory bowel disease
The study showed promising results from different medications for inflammatory bowel diseases such as ulcerative colitis and Crohn's disease. Some monoclonal antibodies and anticytokine molecules have shown clinical remission rates in patients, while small molecules have demonstrated efficacy as induction therapy. Phase 3 studies are underway to validate its efficacy and safety.
Scopus
Shimizu et al. (2019)
Stem cell-based therapy for inflammatory bowel disease
Stem cell-based therapy for inflammatory bowel disease
The results indicated that stem cell-based therapy may be a promising option for the treatment of refractory Inflammatory Bowel Disease (IBD). Hematopoietic stem cell transplantation has been shown to be effective in reducing the need for immunosuppressive medication, but has had serious adverse events. Mesenchymal stem cell transplantation, on the other hand, has shown efficacy in the treatment of complex perianal fistulas, with encouraging results and adverse effects similar to the placebo group.
Scopus
Misselwitz et al. (2020)
Emerging Treatment Options in Inflammatory Bowel Disease: Janus Kinases, Stem Cells, and More
Present the emerging treatment options in inflammatory bowel disease: Janus Kinases and stem cells
The study highlights the development of new therapeutic agents that target selective cytokine inhibition and JAK-STAT signaling for the treatment of inflammatory bowel diseases. These therapies have the potential to improve efficacy and reduce side effects associated with existing treatments. Larger studies are needed to evaluate its long-term efficacy and safety.
Scopus
Schmidt, Grunert and Stallmach (2021)
An update for pharmacologists on new treatment options for inflammatory bowel disease: the clinicians' perspective
Provide an update on new treatment options for inflammatory bowel disease
The study highlights the efficacy of vedolizumab and ustekinumab in the treatment of IBD. Although etrolizumab, AJM300, and ontamalimab have shown positive results in certain patient populations, their overall efficacy and future development require further investigation. Selective blockade of IL-23 with brazikumab, risankizumab, and mirikizumab has shown promise in inducing remission in patients with CD and UC, with optimization of dosing being an important consideration.
Scopus
Yamamoto-Furusho and Parra-Holguín (2021)
Emerging therapeutic options in inflammatory bowel disease
Present the efficacy and safety of new treatments for IBD
The study presented the advances achieved in recent years in the development of new therapies for the treatment of inflammatory bowel diseases. These therapies aim to block inflammation in the gastrointestinal tract, promote mucosal healing, and offer more personalized treatments, aiming at clinical remission and healing of intestinal lesions.
Scopus
Source: Own Authorship (2023).
Table 2: Description of the studies.
Chart 1 presents the main elements of the manuscripts selected for the accomplishment of this review research, which includes the researchers, article title, objectives, methodology, and database.
Discussions
The study by Verstockt et al. (2018) discussed the main studies related to the treatment of inflammatory bowel diseases (IBD) and presented new and promising approaches to this end. One of the approaches highlighted in the study is biological therapies, which involve the use of drugs targeting specific molecules of the immune system to control the inflammatory cascade. These therapies are especially relevant in the treatment of Crohn's disease and ulcerative colitis.
The study highlights the revolution in treatment brought about by the use of tumor necrosis factor inhibitors (anti-TNF's) as a precursor biological therapy, improving quality of life, reducing symptoms, hospitalizations and the need for surgeries. However, some patients do not respond to this therapy, and another group has an initial response with progressive loss of efficacy during treatment. In this sense, new molecules are under development to act on other immunological pathways in order to provide clinical and histological control of the disease.
Other monoclonal antibodies and small molecules with action on the immune system, previously approved for the treatment of other diseases, are mentioned as therapeutic possibilities for inflammatory bowel diseases and are being tested in large studies to prove their effectiveness, as is the case with Ustekinumab , initially approved for the treatment of psoriatic arthritis, has been tested in four large phase II and III clinical trials in patients with Crohn's disease, proving to be effective in inducing and maintaining clinical remission.
The study highlights some classes of especially promising agents, which are: anti-interleukin 12 and 23 agents (anti-IL12/IL23), anti-adhesion molecules (e.g. anti-intergrin A4, S1P receptor antagonist) and inhibitors from Janus Kinase (iJAK)
The study also addresses the involvement of the intestinal microbiota in the development and progression of IBD. It is suggested that modulation of the microbiota through the use of probiotics, prebiotics and fecal microbiota transplantation can be beneficial adjuvant measures for better control of these diseases.
Duijvestein 's research et al. (2018) addresses the main therapeutic advances in the treatment of Inflammatory Bowel Disease (IBD). The study highlights Etrolizumab, a humanized IgG1 monoclonal antibody directed against the β7 subunit of some integrins, which is being evaluated in phase II and III clinical trials for ulcerative colitis. Drugs from the class of S1P receptor modulators were also mentioned, with Ozanimod and Etrasimod being the representatives of this group. Ozanimod has demonstrated efficacy for the treatment of ulcerative colitis in phase II studies and is in phase III trials. Another promising class of drugs according to the study are anti-cytokine antibodies, such as Risankizumab, a humanized monoclonal antibody aimed at blocking Interleukin-23 (IL-23). Randomized, placebo-controlled phase 2 studies have shown efficacy for the treatment of moderate to severe Crohn's disease.
The study carried out by Lee, Park and Park (2018) presented significant results on advances in the treatment of inflammatory Bowel Disease (IBD). Research has highlighted the use of biological therapies, which target inflammatory molecules involved in the disease, reducing inflammation and alleviating symptoms.
In addition, researchers have investigated the effectiveness of anti-adhesion molecules, signaling-blocking molecules, and direct interleukin blockers in inducing and maintaining remission in Crohn's disease and ulcerative colitis. These medications have shown promise in controlling intestinal inflammation and relieving symptoms. The researchers discussed the mechanisms of action of new targeted therapies, such as Janus kinase (JAK) inhibitors that act by inhibiting mainly the JAK 1 and JAK 3 isoforms, as is the case of tofacitinib, a drug that has proven effective in controlling ulcerative colitis. moderate to severe in phase II and III studies, and also efficient in moderate to severe active Crohn's disease in phase II studies.
Another focus of the study was Morgersen, an oligonucleotide that inhibits the production of SMAD7, a protein that is increased in patients with inflammatory bowel disease and is responsible for preventing the action of endogenous anti-inflammatory drugs in the tissue. The drug's efficacy was tested in a phase II multicenter clinical trial that proved its effectiveness, and is currently in phase III study for both Crohn's disease and ulcerative colitis.
The study highlighted the possibility of combined therapeutic approaches for IBD, which involve using different classes of medications together. These combinations aim to improve treatment efficacy and reduce disease progression. However, there are limitations such as the high cost, the greater risk of adverse effects and the availability of little evidence to support the use of combined immunobiological agents. Therefore, the results of this study provide valuable insights for the development of new treatments and therapeutic strategies for inflammatory bowel disease.
In the research by Na and Moon (2019), the results of different drugs in the final stage of development for the treatment of inflammatory bowel diseases, such as Ulcerative Colitis (UC) and Crohn's Disease (CD), were presented. The drugs tested were divided into three main categories: monoclonal antibodies and small molecules.
Among monoclonal antibodies, Etrolizumab, an anti-integrin, was evaluated in a phase II study in patients with moderate to severe ulcerative colitis, where 124 patients previously treated with anti-TNFs had no favorable response. There was a significantly greater clinical response in the Etrolizumab group compared to the placebo group. Phase 3 studies are currently being conducted to compare the effectiveness of this medication with other treatments.
Another monoclonal antibody, SHP-647 (anti-MAdCAM-1), has been tested in separate phase 2 studies in patients with ulcerative colitis and Crohn's disease. Although clinical remission rates were observed in patients with ulcerative colitis at different doses, no therapeutic benefit was demonstrated in patients with Crohn's disease compared to placebo. Phase 3 studies are underway to evaluate the effectiveness of this medication in patients with ulcerative colitis.
In the small molecule group, researchers described the subgroup of anticytokine molecules, in which several drugs were tested as induction therapy in patients with Crohn's disease. Risankizumab had clinical remission rates of 71%, clinical response of 81% and endoscopic remission of 35%. Brazikumab demonstrated a clinical response in 49.2% of patients compared to 26.7% in the placebo group seen in the eighth week of use. The drugs mirikizumab and guselkumab are also being studied, but the results in patients with Crohn's disease have not yet been released. Phase 3 studies are underway to evaluate the effectiveness of these medications in patients with inflammatory bowel disease.
With regard to briakinumab, an anti-IL-12/IL-23, no significant differences in remission induction were observed in patients with Crohn's disease in preliminary phase 2a and 2b studies. To date, no clinical trials for ulcerative colitis have been reported.
Still in the group of small molecules, in the subgroup of signaling molecules, filgotinib demonstrated efficacy in patients with moderate to severe Crohn's disease, with clinical remission rates of 47%. Upadacitinib has also shown efficacy as induction therapy in patients with Crohn's disease. Phase 2 and 3 studies are underway to evaluate its effectiveness in patients with inflammatory bowel disease. A study conducted by Shimizu et al. (2019) investigated the use of stem cell-based therapy for the treatment of Inflammatory Bowel Disease (IBD). Two approaches were analyzed: Hematopoietic Stem Cell Transplantation (HSCT) and mesenchymal stem cell transplantation (MSCT).
In the case of HSCT, the study reported a randomized controlled trial involving patients with refractory IBD. Of the 45 patients undergoing randomization, 23 received HSCT and 22 received conventional treatment. After 12 months of autologous hematopoietic stem cell transplantation, it was observed that 38.1% more patients in the HSCT group were able to stop immunosuppressive medication compared to the control group.
There was a significant improvement in disease activity index (CDAI) and clinical remission. However, the study also reported the frequent occurrence of serious adverse events in the hematopoietic stem cell transplant (HSCT) group, and there was no statistically significant improvement in sustained disease remission at the end of one year. Therefore, it was concluded that HSCT is not an acceptable treatment option for patients with refractory inflammatory Bowel Disease (IBD).
Regarding Mesenchymal Stem Cell Transplantation (MSCT), phase I/II clinical studies were conducted using bone marrow-derived mesenchymal stem cells (BM-TCM) and adipose tissue-derived mesenchymal stem cells (CTAs). In the study with autologous TCM-MO, although a significantly greater efficacy was not observed compared to conventional therapy, allogeneic TCM-MO was effective for patients with luminal IBD and complex perianal fistulas, resulting in a significant reduction in CDAI, clinical remission, endoscopic improvement and healing of fistulas.
The study also mentions a new therapeutic approach called intestinal stem cell transplantation (ISCT), which uses organoids derived from Intestinal Stem Cells (ISCs). ISCT is considered an innovative form of stem cell-based therapy that aims to heal the intestinal mucosa. It delivers proliferative ISCs and functional epithelial cells to the affected region, with the aim of restoring mucosal barrier integrity and reducing inflammation.
Although a clinical trial of Intestinal Stem Cell Transplantation (ISCT) has not yet been conducted, researchers are optimistic that this approach may hold promise for treating Inflammatory Bowel Disease (IBD). It is believed that ISCT can aid in the healing of the intestinal mucosa by delivering proliferative intestinal stem cells and functional epithelial cells to the affected region.
Importantly, to date, these stem cell-based therapies are still in the research phase and are not considered established treatment options for IBD. More clinical studies are needed to evaluate the safety, efficacy, and feasibility of these approaches.
A study by Schmidt, Grunert and Stallmach (2021) analyzed the use of anti-integrin antibodies and IL-23 blockade in the treatment of Inflammatory Bowel Disease (IBD). With regard to anti-integrin antibodies, vedolizumab, an anti-a4β7 integrin monoclonal antibody, has been shown to have a favorable therapeutic impact in inducing clinical remission compared with adalimumab in patients with IBD.
Etrolizumab, which acts on a4β7 integrin and aEβ7 interactions, was effective in inducing remission in patients with symptomatic Ulcerative Colitis (UC) who had not previously received anti-TNF treatment. However, its efficacy in maintaining remission and treating UC patients who had previously been treated with anti-TNF was less convincing.
An additional option is AJM300, an oral molecule that blocks a4 integrin, and has demonstrated significant rates of clinical response and remission in patients with moderately active Ulcerative Colitis (UC). Regarding ontamalimab (PF-00547659), which acts on MadCAM-1, it was observed that it is superior to placebo in inducing clinical remission in patients with UC, but does not have the same effect in cases of Crohn's Disease (CD) moderate to severe.
It is important to highlight that the efficacy and future development of etrolizumab are still uncertain due to the mixed results obtained in several studies. With regard to blocking IL-23, ustekinumab, an anti-p40 monoclonal antibody that acts on both IL-12 and IL-23, has demonstrated proven efficacy and safety in the treatment of patients with CD and UC.
On the other hand, briakinumab, another anti-p40 monoclonal antibody, did not achieve the primary endpoint of clinical remission in a study of patients with CD. Brazikumab (MEDI2070) and risankizumab, both selective anti-p19 monoclonal antibodies, showed promising results in inducing clinical remission in patients with CD. Furthermore, risankizumab also achieved clinical and endoscopic remission during treatment maintenance. In the case of mirikizumab, an anti-p19 monoclonal antibody, significant rates of clinical response and remission were observed in patients with UC and CD. Additional studies are needed to determine the optimal dosing schedule for induction therapy.
Another therapeutic approach studied was the inhibition of JAK-STAT signaling, using Janus kinase (JAK) inhibitors. Tofacitinib, a JAK1/JAK3 inhibitor, was approved for the treatment of ulcerative colitis, showing efficacy in this condition. Furthermore, filgotinib, a selective JAK1 inhibitor, has also demonstrated efficacy in the treatment of Crohn's disease. Other JAK inhibitors, such as upadacitinib and TD-1473, have shown promising results in the treatment of IBD, offering additional therapeutic alternatives.
The study conducted by Yamamoto-Furusho and Parra-Holguín (2021) aimed to present the main results obtained in the development of new therapies for the treatment of Inflammatory Bowel Diseases (IBD), with a specific focus on blocking Tumor Nnecrosis Factor alpha (TNF-a) and markers of response to treatment.
Anti-tumor necrosis factor alpha therapy has been shown to be effective for patients with IBD who do not respond to conventional treatment. However, it is necessary to develop new therapies and identify markers that can predict response to treatment. In this sense, several pathways involved in the development of IBD have been studied, and new therapies seek to block the inflammatory process in the gastrointestinal tract through different forms of administration, such as oral, intravenous, subcutaneous and topical.
One of the advantages of these new therapies is the possibility of offering more personalized treatments, with higher success rates and fewer relapses. Furthermore, these treatments are not only limited to clinical remission, but also aim to achieve macroscopic changes in the mucosa through healing. This means that treatments aim to promote the healing of intestinal injuries not only in external appearance, but also at a microscopic level, restoring the integrity of the mucosa.
Treatments under development are mainly based on modifying signaling pathways involved in inflammation, blocking specific receptors or ligands, reducing cell migration and preserving the integrity of the epithelial barrier. These approaches have shown efficacy and safety in studies carried out to date.
Final Considerations
This integrative review explored new therapeutic options for IBD and their effectiveness in controlling symptoms and improving patients' quality of life. Based on the analysis of the reviewed studies, it can be concluded that several therapies have demonstrated promising results in the treatment of IBD.
It is possible to state that the treatment of IBD has evolved significantly in recent years. Several therapeutic approaches have shown promise in reducing inflammation and relieving symptoms associated with Crohn's disease and ulcerative colitis.
Biological therapies have gained prominence as an effective option in the treatment of IBD. These medications target specific immune system molecules, such as cytokines and integrins, to reduce intestinal inflammation. Monoclonal antibodies have been shown to be effective in controlling inflammation and promoting intestinal healing.
Other therapeutic approaches, such as modulation of the intestinal microbiota through probiotics, prebiotics and fecal transplantation, have also been explored as an effective strategy in the treatment of IBD. Research indicates that manipulating the microbiota can play an important role in improving symptoms and reducing intestinal inflammation.
In addition to established therapies, new classes of medications are being studied, such as Janus kinase inhibitors and cell adhesion molecule inhibitors. These therapies target specific targets involved in inflammation and have shown potential in controlling the disease. Combining different drug classes has been explored as a strategy to improve treatment efficacy and reduce disease progression.
In the field of stem cell-based therapies, there is still much to be explored. Although preliminary studies have shown promising results with hematopoietic stem cell transplantation and mesenchymal stem cell transplantation, more research is needed to evaluate the safety, efficacy, and feasibility of these approaches. Intestinal stem cell transplantation is a new therapeutic approach that may have the potential to promote healing of the intestinal mucosa, but still requires clinical trials to validate its effectiveness.
References
- De Barros GVN, Silva TS, De Santos Oliveira Brito AP, Garcia HCR, Maneschy RB. Métodos diagnósticos e terapêuticos das doenças inflamatórias intestinais: revisão sistemática. Pará Res Med J. 2020; 4: 0.
- Belém M, Oda JY, Yasuo J. Doenças inflamatórias intestinais: considerações fisiológicas e alternativas terapêuticas. Arq Cienc Saúde Unipar. 2015; 19: 73-9.
- Brasil, Lei n. 9.610, de 19 de fevereiro de 1998. Altera, atualiza e Consolida a legislação sobre direitos autorais e dá outras providências. Diário Ofi¬cial da união, Brasília, DF. 1998.
- Bruchez A, d’Avila AAF, Fernandes AM, Castilhos NC, Olea PM. Metodologia de pesquisa de dissertações sobre inovação: análise bibliométrica. Desafio on line, Caxias do Sul-RS. 2015; 6: 1-14.
- Cambui YRS, Natali MRM. Doenças inflamatórias intestinais: revisão narrativa da literatura. Rev Fac Cienc Med Sorocaba. 2015; 17: 116-9.
- Catalan-Serra I, Brenna ø. Immunotherapy in inflammatory bowel disease: novel and emerging treatments. Hum Vaccin Immunother. 2018; 14: 2597-611.
- Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci. 2017; 38: 127-42.
- Duijvestein M, Battat R, Vande Casteele N, D’Haens GR, Sandborn WJ, Khanna R et al. Novel therapies and treatment strategies for patients with inflammatory bowel disease. Curr Treat Options Gastroenterol. 2018; 16: 129-46.
- Ellinghaus D, Bethune J, Petersen BS, Franke A. The genetics of Crohn’s disease and ulcerative colitis–status quo and beyond. Scand J Gastroenterol. 2015; 50: 13-23.
- Guan Q. A comprehensive review and update on the pathogenesis of inflammatory bowel disease. J Immunol Res. 2019; 32: 1-16.
- Hazel K, O’Connor A. Emerging treatments for inflammatory bowel disease. Ther Adv Chronic Dis. 2020; 11: 2040622319899297.
- Kaplan GG. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017; 152: 313-321.e2.
- Khan I, Ullah N, Zha L, Bai Y, Khan A, Zhao T, et al. Alteration of gut microbiota in inflammatory bowel disease (IBD): cause or consequence? IBD treatment targeting the gut microbiome. Pathogens. 2019; 8: 126.
- Koche JC. Fundamentos de metodologia científica. Editora vozes; 2016 [cited janv 23 2023]. Available from: https://btux.com.br/professorbruno/wp-content/uploads/sites/10/2018/07/K%C3%B6che-Jos%C3%A9-Carlos0D0AFundamentos-de-metodologia-cient%C3%ADfica-_-teoria-da0D0Aci%C3%AAncia-e-inicia%C3%A7%C3%A3o-%C3%A0-pesquisa.pdf.
- Lee HS, PARK SK, PARK DI. Novel treatments for inflammatory bowel disease. Korean J Intern Med. 2018; 33: 20-7.
- Minayo MCSO desafio do conhecimento: Pesquisa qualitativa em saúde. São Paulo: Hucitec. 2013.
- Misselwitz B, Juillerat P, Sulz MC, Siegmund B, Brand S, et al. An official working group of the Swiss Society of Gastroenterology. Emerging treatment options in inflammatory bowel disease: Janus kinases, stem cells, and more. Digestion. 2020; 101: 69-82.
- NA SY, Moon W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver. 2019; 13: 604-16.
- Prodanov CC. FREITAS, Erna ni Cesar. Metodologia do trabalho científico: métodos e técnicas da pesquisa e do trabalho acadêmico-2ª Edição. Ed Feevale. 2013.
- Ramos GP, Papadakis KA. Mechanisms of disease: inflammatory bowel diseases. Mayo Clin Proc. 2019; 94: 155-65.
- Roman AR, Friedlander MR. Revisão integrativa de pesquisa aplicada à enfermagem. Cogitare Enferm. 2018; 3.
- Santos LAA, Dorna MdS, Vulcano DSB, Augusti L, Franzoni LdC, et al. Terapia nutricional nas doenças inflamatórias intestinais: artigo de revisão Soc. Bras. Aliment Nutr. 2015; 40: 383-96.
- Santos LMT, Fernandes IMR. Qualidade de vida da pessoa com doença inflamatória intestinal. Rev Enferm Ref. 2019; 4: 89-98.
- Schmidt C, Grunert PC, Stallmach A. An update for pharmacologists on new treatment options for inflammatory bowel disease: the clinicians’ perspective. Front Pharmacol. 2021; 12: 655054.
- Seyedian SS, Nokhostin F, Malamir MD. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J Med Life. 2019; 12: 113-22.
- Shimizu H, Suzuki K, Watanabe M, Okamoto R. Stem cell-based therapy for inflammatory bowel disease. Intest Res. 2019; 17: 311-6.
- Tontini GE, Vecchi M, Pastorelli L, Neurath MF, Neumann H. Differential diagnosis in inflammatory bowel disease colitis: state of the art and future perspectives. World J Gastroenterol. 2015; 21: 21-46.
- Vasconcelos RS, Rocha R, Souza E, Amaral V. Qualidade de vida de pacientes com doença inflamatória intestinal: revisão integrativa. ESTIMA Braz J Enterostomal Ther. 2018; 16: e2118.
- Verstockt B, Ferrante M, Vermeire S, Van Assche G. New treatment options for inflammatory bowel diseases. J Gastroenterol. 2018; 53: 585-90.
- Verstockt B, et al. Novas opções de tratamento para doenças inflamatórias intestinais. Rev Gastroenterol. 2018; 53: 585-90.
- Yamamoto-Furusho JK, Parra-Holguin NN. Emerging therapeutic options in inflammatory bowel disease. World J Gastroenterol. 2021; 27: 8242-61.