
Research Article
Austin J Gastroenterol. 2025; 12(1): 1132.
The Interaction between Diesel Exhaust Exposure and High -Fat Diet in the Insulin Resistance
Hemmatpour A1, Ghaneie S1, Momen A2, Sakhvidi MJZ2, Karimollah A3, Nemati M4 and Reza JZ1*
1Department of Clinical Biochemistry, School of medicine, Shahid Sadoughi University of medical sciences and Health Services, Yazd, Iran
2Department of Occupational Health, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
3Department of Pharmacology, School of Pharmacy, Shahid Sadoughi University of Medical Sciences and Health Services, Yazd, Iran
4Endocrinology and Metabolism Research Center, Shiraz University of Medical Sciences, Shiraz, Iran
*Corresponding author: Javad Zavarreza Reza, Department of Clinical Biochemistry, School of medicine, Shahid Sadoughi University of medical sciences and Health Services, Yazd, Iran Tel.: +98 (35) 3725 8410; Fax: +98 (35) 3725 4750; Email: jzavar@ssu.ac.ir
Received: May 22, 2025 Accepted: June 13, 2025 Published: June 17, 2025
Abstract
Numerous investigations have disclosed the factors involved in type 2 diabetes; one of the them is a high-fat diet. Researchers have revealed that exposure to particulate matter (PM) can increase the risk of insulin resistance and diabetes; however, the mechanisms involved are still unclear. Incretin hormone receptors (GLP-1R and GIPR) and transcription factor 7-like 2 (TCF7L2) are key players in nutrient-induced insulin secretion when nutrients are taken in. We hypothesized that particulate matter (PM) could trigger insulin resistance by interfering with the above mechanisms.
Thus, we study the role of pancreas malfunction as well as the interaction between diesel exhaust (PM) and high-fat diet (HFD) in the development of insulin resistance. Four groups of C57BL/6 mice (namely N/F, H/F, N/P, and H/P) were studied.
Mice fed the standard, HFD and then exposed them to PM-filtered air for 10 weeks. We conducted the expression of Gipr, Glp-1r, and Tcf7l2 (variant E4) genes, as well as the expression of TCF7L2 proteins. Insulin surrogate indices evaluated blood glucose and insulin sensitivity. We also performed blood lipid profiling and liver function tests. Despite maintaining their insulin tolerance, mice fed a lot of fat showed lower levels of GIPR and pancreatic TCF7L2 proteins, a sign of poor glucose tolerance. PM exposure decreased Gipr, Glp-1r, and Tcf7l2-E4 expression; however, glucose tolerance and insulin sensitivity did not show any significant change.
Moreover, PM significantly increased the levels of pancreatic TCF7L2 protein. New research shows that PM short-term exposure can alter the way genes work that control insulin production and release. This could cause glucose and insulin intolerance if it lasts for a long time.
Keywords: Gipr; Glp-1r; Tcf7l2; C57BL/6 mice; Air pollution; High-fat diet
Introduction
Various environmental factors can affect epigenetic regulation of metabolism, suggesting that metabolic diseases are becoming more prevalent as a result of environmental variables [1]. Type-2 diabetes (T2DM) is a multifactorial disorder that may result from a lifestyle, diet, or genetic susceptibility. However, the evidences Show that environmental pollution remains a missing piece in the disease etiology puzzle. The effect of exposure to air pollution on the chance of getting T2DM has been investigated in various studies, and it has been shown that ambient air pollution has an important role in the insulin resistance induction [2,3]. The main part of air pollution is Particulate matter (PM2.5, PM10, and smaller sizes), which can potentially contribute to the development T2DM, even at low levels [4]. According to recent studies, this can be even more threatening when accompanied by other risk factors, such as a high-fat diet (HFD) [5]. Researchers have proposed some biological factors (inflammation, oxidative stress) to explain this finding. However, due to the intricacy of diabetes etiology and the chemical composition of air pollutants, the underlying mechanism remains largely unknown [6,7].
The canonical pathway, β-catenin/TCF7L2-dependent Wnt signaling, which orchestrates several key regulators, has a pivotal role in normal insulin synthesis and secretion [8,9]. The islets in the pancreas make insulin, glucagon, and the incretin hormones GLP-1 and GIP. These hormones are crucial for keeping glucose levels stable. Primarily, the pancreatic islets express incretin hormones, which regulate glucose levels through their respective receptors. Potential disruptors of TCF7L2, a functional element of incretin hormones and a regulator of their receptors in β-cells, interact with incretin receptors, which can impair beta -cell function and lead to diabetes [10].
Studies have indicated an intricate and tissue-specific splicing pattern for Tcf7l2 with distinct functions. Exon-4-contained variants (Tcfl2-E4) are the most common in pancreatic cells. They are also known to have inhibitory functions that influence both insulin synthesis and β-cell survival [11,12,13].
A lot of research has been done on the Tcf7l2 gene polymorphism and how it affects glucose homeostasis. However, earlier research didn't find a link between the genotype that makes someone more likely to get T2D and differentially spliced Tcf7l2 transcripts [12,14]. A study conducted by Shu et al. (2009) showed that Tcf7l2 expression declined in high-fat-fed mice, making us wonder whether the flexible frame of splicing and alterations in this gene expression have a critical role in the pancreas response to metabolic stress [15].
Several studies have highlighted PM-mediated T2DM due to its epigenetic dysregulation outcomes [16]. However, few researches have examined the direct effect of PM on the expression of critical pancreatic regulatory genes involved in insulin synthesis and secretion. Furthermore, in earlier research, the obesogenic diet intervention usually came before PM exposure. This study, on the other hand, looked at how metabolic challenges might work together in a synergistic way.
Methods
Animal Care
Royan Laboratories (Isfahan, Iran) provided male C57BL/6 mice (8-12 weeks). We kept the mice on a 12:12-hour light-dark cycle and free access to water and food. The Medical Ethics Committee of Shahid Sadoughi University approved the study (Registration Code: IR.SSU.MEDICINE.REC.1395.244).
Study Design
Following a 12-day acclimatization period in the experimental chamber, we randomly assigned mice to four groups: control (N/F, n = 10), PM (N/P, n = 10), HFD (H/F, n = 10), and HFD + PM (H/P, n = 10). The N/F and N/P groups fed their animals a standard chow diet for 10 weeks, while the H/F and H/P groups fed them a diet containing 45 percent fat calories. For 10 weeks, we exposed the mice to either PM or HEPA-filtered air (FA) for 6 hours per day, 6 days per week. Throughout the study, we weighed the animals every two weeks. The exposure occurred during the light cycle. Blood glucose levels were measured weekly after 6-8 h of fasting by the SD code-free glucometer (SD BIOSENSOR, Inc., Korea) (5 μL of blood).
PM Exposure and Monitoring
A well-sealed glass chamber with constant airflow (6 m/h) housed the mice. A sucking pump injected particulate matter emissions from a light-duty diesel engine into the experimental chambers. We obtained real-time data on emitted PM by a monitoring system (Environmental Dust Monitor 365, GRIMM, Grimm Aerosol Technik GmbH & Co., KG, Ainring, Germany). Hourly particle number concentrations (PNCs) were measured with size distributions of 0.25, 10, and 1 μm. We also performed a zero check on the control cages.
Euthanasia, Blood Sampling, and Tissue Collection
We fasted the mice for 10-13 hours 24 hours after the last PM exposure, anesthetized them with thiopental (50 mg/kg, i.p.), and euthanized them by cervical dislocation [17]. We used serum separator tubes (SSTs) to obtain blood samples through cardiac puncture. We refrigerated the tubes overnight, centrifuged them at 2000 g for 15 minutes, and then stored them for further analysis. We quickly dissected and split the pancreas into two halves from the head to the tail. We stored one half for ELISA, while we minced the other, immersed it in RNA later and stored at -80°C [18,19].
Lipid Parameters
We analyzed the lipid profiles in blood samples for 10 weeks to assess the influence of inhalation exposure to PM on lipid homeostasis. We measured HDL-C, TG, and TC levels using commercial kits (Pars Azmun, Iran). We calculated the LDL-C level using the Friedewald formula [20].
Liver Function Test
After 10 weeks of intervention, the available commercial kit (Pars Azmun, Iran) measured the levels of ALT, AST and ALP to assess the hepatic function status.
Serum/Pancreatic Insulin and TCF7L2 Protein Assessment
Half of the pancreas sample, allocated for protein quantification, was rinsed, suspended, and homogenized in ice-cold PBS (1X). The suspension was sonicated three times on ice and centrifuged, and the supernatant used for ELISA. The pancreatic TCF7L2 protein assay was performed using a mouse transcription factor 7-like 2 ELISA kit (My Bio Source Inc., San Diego, CA). Serum/tissue total insulin content was measured by using ELISA kit (Abnova, Taiwan). The amount of insulin in the pancreas was compared to the total protein content of the tissue (the Bradford assay was used to measure pancreatic total protein) [21]. Insulin sensitivity status was also assessed by calculating surrogate indices (log HOMA-IR, QUICKI, %B, %DI, %S).
Analysis of Gene Expression by Quantitative PCR
Total RNA was isolated from the pancreas using the TRIZOL Reagent (Life Technologies, Carlsbad, CA) in combination with the Total RNA extraction kit (Vivantis Technologies, Malaysia) [22]. cDNA was synthesized from 1 μg of mRNA using a High-Capacity cDNA Reverse Transcriptase Kit (Thermo Fisher Scientific, Vilnius, Lithuania). Quantitative real-time PCR analysis on a Light Cycler 96 System (Roche Applied Science, Indianapolis, IN, USA) using standard procedures. The SYBR Green I Master Kit (Amplicon, Odense M, and Denmark) amplified the target genes. The primers used for real-time PCR. We determined the fold changes in mRNA levels after normalizing them to the internal control β-actin mRNA levels.
Statistical Analysis
We analyzed the data using SPSS19.0 IBM statistics software and presented the results as means and SEM. To normalize the nonnormally distributed data, we log10 transformed β%, DI%, and S% values. We performed a repeated measures analysis for the weekly weight and serum glucose measurements. We used the General Linear Model (GLM) univariate analysis, with Bonferroni's adjustment for pairwise comparisons, to assess the interaction between PM and HFD as fixed factors. Statistical significance was considered at p < 0.05.
Results
Exposure Characterization
As illustrated in Figure 1A, PM exposure was constant during the study. The mean concentration of PM in the exposure chamber during the 10 weeks of exposure was 120 μg.m-3 (~10-fold higher than the ambient PM concentration).
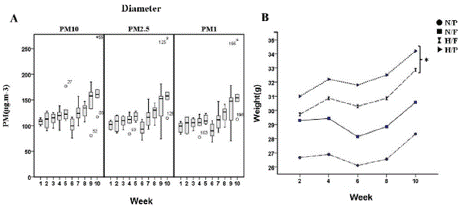
Figure 1: (a): The particulate matter levels (in diameter) in the
exposure chamber and (b) Mice weight gain during the study (* P = 0.019
Vs. control). The results are means ± S.E.M.
Figure 1 Alt Text: Figure 2 shows A) A box plot depicting the particulate
matter levels (by diameter) in exposure chambers on the y-axis against
weeks on the x-axis. B) A line chart showing mice weight (on the Y-axis) by
group name against week (on the X-axis).
Bodyweight Investigations
Figure 1B depicts the mean weight in the experimental groups. During the study, high-fat-treated mice significantly gained weight compared to the control group, whereas PM-exposed mice showed no significant weight gain (p = 0.963). GLM univariate analysis did not reveal a significant interaction between PM exposure and HFD (p = 0.160).
Fasting Blood Glucose, Fasting Serum, and Pancreatic Insulin Levels
FBG was monitored weekly during the study and data showed FBG levels in the PM-exposed mice were similar to the FA-exposed mice. However, FBG levels rose significantly in high-fat-fed mice at weeks 7 and 10 compared with the control (Figure 2A-D). The PMinhaled groups (p = 0.351) and HFD groups (p = 0.330) did not significantly alter their serum levels of insulin after 10 weeks (Figure 2E).
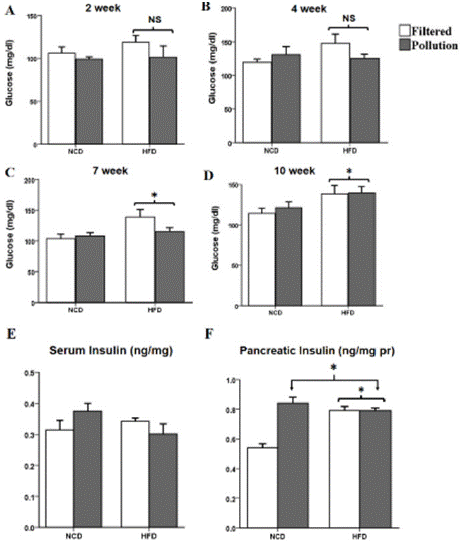
Figure 2: Fasting blood glucose and insulin levels. FBG levels followed an
upward trend in 6-8 h fasted mice during the first weeks on a high-fat diet
(a) after 2 weeks (* P= 0.42 vs. control) and (b) after 4 weeks (* P= 0.29 Vs.
control) but reached significance after (c) 7 weeks (* P= 0.011 Vs. control).
However, the difference did not remain significant until (d) 10 weeks on the
diet when FBG increased significantly in HFD groups compared to the control
(* P= 0.037 Vs. control). (e) Serum insulin levels, and (f) Pancreatic insulin
content in PM- exposed (* P = 0.01 vs. control) and HFD-treated (* P= 0.004
Vs. control) mice. The results are means ± S.E.M
Figure 2 Alt Text: Figure 2 shows clustered bar charts grouped according to
the diet (high fat or normal) and air quality (polluted or clean) depicting A-D)
glucose levels on weeks 2, 4, 7, and 10, respectively. E) Serum insulin levels
among the groups at the end of the study F) Bar chart depicting pancreatic
insulin levels at the end of the study. Filtered air groups are white bars, and
polluted air groups are gray.
However, the pancreatic insulin content increased in both the PM- and HFD groups (Figure 2F). The GLM univariate analysis revealed a significant interaction between PM exposure and the HFD effect on pancreatic insulin levels (p < 0.001).
Effect of Sub-Acute PM Exposure on Glucose Tolerance and IR
We generated surrogate indices of insulin resistance and sensitivity based on the FBG and insulin levels in blood drawn before euthanasia. We adjusted the indices for body weight and found no significant change in the insulin sensitivity state of the groups [23,24].
Sub-Acute Exposure to PM Altered Serum Lipid Profile
The serum TG levels were comparable between the control and experimental groups (Figure 3A). Serum TC levels increased significantly in the HFD groups but did not reach significance in PMexposed mice (p = 0.496) (Figure 3B). As shown in Figure 3C, D, mice fed an HFD showed a marked increase in serum HDL-C and LDL-C levels, while PM-inhaled mice showed no alteration (HDL, p = 0.168; LDL, p = 0.113). In comparison to the control group, the HDL/LDL ratio decreased significantly in all three groups (Figure 3E).
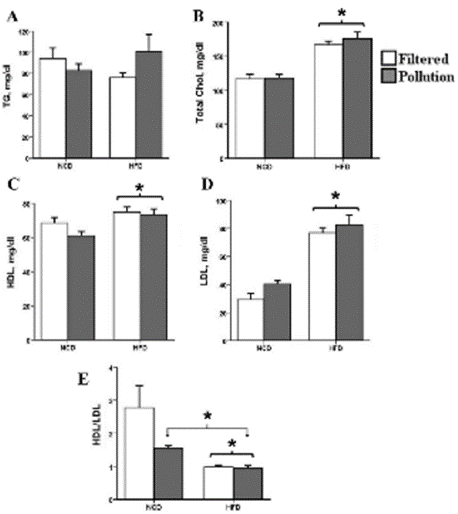
Figure 3: Effect of PM exposure and high- fat feeding on Lipoprotein profile.
(a) TG (b) Total cholesterol (* P = 0.01 Vs. control) (c) HDL (* P = 0.007 Vs.
control) (d) LDL (* P < 0.001 Vs. control), and (e) HDL/LDL ratio (* P = 0.01
Vs. control). The results are means ± S.E.M.
Figure 3 Alt Text: Figure 3 shows clustered bar charts grouped according
to the diet (high fat or normal) and air quality (polluted or filtered) depicting
A-E) serum lipid parameters levels, including triglyceride, total cholesterol,
HDL-C, LDL-C, and HDL/LDL ratio at the end of the study. Filtered air
groups are white bars, and polluted air groups are gray.
Sub-Acute PM Inhalation Disrupts Hepatic Function
AST, ALT, and ALP levels increased significantly after 10 weeks of exposure in the PM-treated mice (Figure 4A-C). Nevertheless, enzyme levels did not change in the HFD groups (AST, p = 0.242; ALT, p = 0.466; ALP, p = 0.429). The mice's serum lipid profile, elevated hepatic enzymes, and inflammatory status (data not shown) indicate the early onset of NAFLD in the PM-inhaled groups.
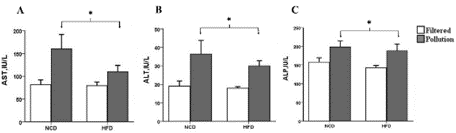
Figure 4: Effect of PM exposure and high -fat feeding on Serum hepatic
enzymes (a) AST (* P = 0.019 Vs. control) (b) ALT (* P = 0.007 Vs. control),
and (c) Alp (* P = 0.012 Vs. control). The results are means ± S.E.M.
Figure 4 Alt Text: Figure 4 shows clustered bar charts grouped according
to the diet (high fat or normal) and air quality (polluted or clean) depicting
A-C) serum hepatic enzymes, including AST, ALT, and ALP, respectively.
Filtered air groups are white bars, and polluted air groups are gray.
PM Inhalation Associated with A High-Fat Diet Decreases Incretin Receptor Expression
The gene expression of Gipr declined considerably in both HFD-fed mice and PM-exposed groups (Figure 5B). Glp-1r mRNA expression was also decreased relative to β-actin in the PM-exposed groups. Meanwhile, the high-fat-fed counterparts did not show a significant difference compared with the control (p = 0.225) (Figure 5A). The GLM univariate analysis also revealed a significant interaction between PM exposure and HFD effect on the expression of Gipr (p = 0.016).
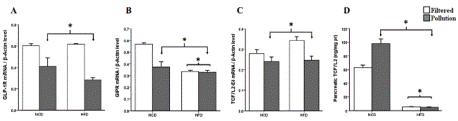
Figure 5: Effect of PM exposure and high- fat diet on the pancreatic
expression of (a) Glp-1r (* P = 0.01 Vs. control) (b) Gipr in PM exposed (*
P= 0.011 Vs. control) and HFD treated (* P= 0.001 Vs. control) mice, (c)
Tcf7l2-e4 (* P= 0.007 Vs. control) (d) TCF7L2 protein content in PM exposed
(* P = 0.01 Vs. control) and HFD treated (* P = 0.01 Vs. control) mice. The
results are means ± S.E.M.
Figure 5 Alt Text: Figure 5 shows clustered bar charts grouped according to
the diet (high fat or normal) and air quality (polluted or clean) depicting A-C)
mRNA expression levels of GLP-1R, GIPR, and TCF7L2-E4 D) pancreatic
TCF7L2 protein content. Filtered air groups are white bars, and polluted air
groups are gray.
Sub-Acute PM Inhalation Effect on Tcf7l2 Expression
HFD hadn't significant effect on the level of Tcfl2-E4 (p = 0.118), but it did drop significantly in groups exposed to PM compared to the control (Figure 5C). This study also calculated the pancreatic TCF7L2 protein content to examine the correlation between the expression of the E4 variant of Tcfl2 and its protein expression. The amount of TCF7L2 protein dropped a lot in the groups that were fed a lot of fat, but it went up when they were exposed to PM (Figure 5D).
Discussion
The main finding of our study was that short-term exposure to diesel exhaust particulate matter could cause pancreatic dysfunction by messing up the way genes are expressed normally in the pancreas. The present findings also suggest that exhaust PM exposure may enhance the risk of 2DM by promoting insulin-induced insulin resistance.
Numerous lines of records discuss the close association, between the TCF7L2 and T2DM, but it's unclear how diabetogenic environments affect its expression levels. We found that people on high-fat diets had less TCF7L2 protein in their pancreas, but their Tcf7l2-E4 mRNA levels didn't change much. Previous findings regarding the Tcf7l2 gene and protein expression under a high-fat diet are controversial. In this case, Shu et al. showed that the TCF7L2 protein level dropped even though the gene level went up after eating an HFD, while Hu et al. found that both the Tcf7l2 gene and protein expression went down [15,25]. However, Yang et al. (2012) reported an increased TCF7L2 protein expression in mouse pancreas fed on an HFD [26]. Later, in 2012, Shu et al. reported that TCF7L2 protein expression followed a phasic pattern under an HFD that increased in the early weeks of exposure and decreased when hyperglycemia became apparent. They concluded that TCF7L2 levels are associated with β-cell compensation during a HFD [27]. Also, Tcf7l2+/- mice had lower levels of insulin and glucose in their blood, better glucose tolerance, and higher insulin sensitivity when fed normal food or an HFD compared to their wild-type littermates. This made them less likely to get diabetes from their food [26,28]. However, both mild and acute specific Tcf7l2 depletion from the pancreas resulted in impaired glucose tolerance, while the islet and plasma insulin content remained comparable to the control group [29,30]. The most common type of TCF7L2 isoform in pancreatic islets is the one that contains exon- 4 and has a lower ability to transactivate genes [12]. No data are available on dietary-mediated alterations in the expression of exon 4-containing isoforms. A study by Pradas-Juni et al. (2014) on human B lymphocytes was interesting because it showed that exon 4 was more likely to be included in T2D patients with the at-risk T/T genotype, but less likely to be included in non-diabetic at-risk T/T carriers [31]. When mice were exposed to PM, their Tcf7l2-E4 levels went down, which was different from the HFD groups in this study, and based on their metabolic traits, the higher exclusion of exon 4 might help protect them against metabolic stress. Pradas-Juni et al. (2014) also found reduced TCF7L2 protein levels in patients with diabetes with an at-risk T/T genotype [31]. The present results showed a reciprocal pattern in the alterations of Tcf7l2-E4 and TCF7L2 protein expression under both HFD treatment and PM exposure, indicating that exon 4-containing transcripts may further suppress the transcription of Tcf7l2.
Assessment of mRNA expression also revealed a decline in Gipr mRNA levels in HFD. Some studies have extensively documented a decreased incretin effect in diabetes mellitus, particularly in GIP [32,33]. Later research showed that the loss of the incretin effect was caused by lower expression of incretin receptors in pancreatic islet cells [34].
Recent data suggests that GIP is responsible for fat intake, while GLP-1 is associated with excess caloric intake, which could explain the greater reduction in Gipr in HFD groups [35].
Since an HFD is one of the major factors of T2DM, more research needs to be done on how incretin receptors affect glucose homeostasis in HFD-induced metabolic dysmetabolism. GIP receptor antagonism counters obesity and insulin resistance in HFD mice, according to McClean et al. and others [36,37]. Another thing is that mice lacking Glp-1r and Gipr (Gipr -/-, Glp-1r -/-) and double incretin receptors are fed high-fat diets and are attacked with cytokines. These mice don't get fat or develop insulin resistance [21,38,39].
Several researchers have also proposed that β-cells have selfadaptive tricks during diabetes progression. In this line, Kubo et al. showed that restoring Glp-1r expression in diabetic db/db mice makes glucose intolerance worse compared to db/db control mice. They came to the concluded the different effects of Glp-1r activity on β-cell functions in diabetic and non-diabetic mice [40]. In line with their findings, Srivastava et al. said that the slowed- down incretin response is part of a two-phase pattern of expression that happens before T2DM develops. It may be a way for the pancreas to deal with stress and keep glucose tolerance normal [41].
Previous studies reported increased serum and pancreatic insulin in response to an HFD [25,37]. Given that disrupted incretin is one of the early markers of β-cell dysfunction, this reduction leads to impaired insulin secretion and hyperinsulinemia, as evidenced by the elevated FBG despite higher pancreatic insulin and preserved insulin sensitivity in the HFD groups [42]. We hypothesize that prolonged exposure to excess nutrients could override the compensatory effect of receptor loss.
The aforementioned studies suggest that the response to HFD is a phasic process, with glucose intolerance peaking within three days and remaining in the plateau phase between weeks 1-12 [43].
In agreement, Mosser et al. (2015) reported that fasting insulin and insulin tolerance remain unchanged until the 11th week, when elevated fasting insulin and insulin intolerance become apparent despite early impaired glucose tolerance in HFD mice [44]. Therefore, the present study's observation of normal insulin tolerance could be the result of testing during the stable transient phase of insulin resistance development. This could be due to the relatively higher fat content of the control diet compared with similar studies (~14%). In studies using the HFD-induced diabetes methodology, the administered diet fat content is normally 4–10 times higher than that of the control, which is 3.5 times in this study.
These results are the first to show that short-term exposure to PM hurts the function of the pancreas. This is likely because it lowers the expression of Glp-1r, Gipr, and Tcf7l2, which can make insulin resistance worse. We detected no significant changes in fasting blood insulin (despite the upward trend), fasting blood glucose, or body weight (despite the downward trend) in the PM-exposed groups. According to the literature, PM exposure can increase serum insulin levels in mice and eventually lead to insulin resistance, though almost no data exist on pancreatic insulin content [4,45,46]. However, Miranda et al. (2018) reported that maternal exposure to PM increases islet insulin content in the offspring, a finding that aligns with current findings [47]. Recently, Bosch et al. (2019) showed that intratracheal exposure of mice to PM did not affect glucose tolerance, insulin, body weight, or fasting glucose, whereas oral administration of PM impaired glucose tolerance, decreased insulin, and hadn't effect on the body weight, FBS, or insulin sensitivity [48]. They took out pancreatic Β-cells and found that the islet insulin content didn't change much (even though it was going up), but the number of small islets was slightly higher in the mice that were given the drug by mouth [48]. In this study, insulin sensitivity was about the same as the control group, but serum AST and ALT levels went up a lot in the PM-exposed groups, and the HDL/LDL ratio went down a lot, which may make the risk of NAFLD higher [49].
Documents showed that PM exposure elevates the risk of fatty liver disease [45,46]. High levels of transaminase correlate to insulin resistance and T2DM [50,51]. Higher levels of hepatic enzymes, alkaline phosphatase, and NAFLD are independent predictors of T2DM. These levels often show up before other insulin resistance mediators do [50,52,53].
We found no significant evidence of their synergistic effect on glucose tolerance and insulin resistance indices when we administered PM and HFD simultaneously. However, we observed a synergistic effect on pancreatic insulin, TCF7L2 protein, and Gipr expression. Xu et al. (2010) looked into how PM could cause insulin resistance to develop during the same period, time but with only concentrated PM2.5 exposure. They did not find that HFD and PM exposure had any synergistic effects on glucose tolerance and HOMA-IR [54]. Still, giving HFD and PM2.5 together (through intranasal instillation) in a recent study made people less able to handle glucose, but only after 12 weeks of the study [55].
Conclusion
PM inhalation may make it harder for the pancreas to respond properly to insulin needs. This, along with insulin resistance in the liver, can make it harder for the body to handle glucose, which can eventually lead to 2DM. Our study also showed that distinct metabolic stresses can influence islet gene expression, and metabolic stress can alter TCF7L2 and incretin receptor expression long before insulin resistance develops.
Acknowledgment
The authors appreciate Y. Vahidi, M. Momtaz, F. Zare, S. Hosseini, Z. Hafizi, V. Dashti, and S. Dastgheib for their support in conducting this study.
Availability of Data and Materials
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request.
Ethical Approval
Medical Ethics Committee Registration Code: IR.SSU. MEDICINE.REC.1395.244.
Consent to Participate
All the authors expressed their consent to cooperate in studying and writing the article.
Authors Contributions
Dr. Mohammad Javad Zare Sakhvidi is the supervisor for environmental air pollution, Dr. Javad Zavarreza is the supervisor for molecular and biochemical, Dr. Alireza Karimollah for animal treatment, Anahid Hemmatpour and Sima Ghaneie and Marzieh Nemati for practical prat. All authors contribute in writing the article.
References
- Wu, Y.L., Lin, Z.J., Li, C.C., Lin, X., Shan, S.K., Guo, B., et al. Epigenetic regulation in metabolic diseases: mechanisms and advances in clinical study. Signal Transduct Target Ther. 2023; 8: 98.
- Dang, J., Yang, M., Zhang, X., Ruan, H., Qin, G., Fu, J., et al. Associations of Exposure to Air Pollution with Insulin Resistance: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2018; 15.
- Rao, X., Montresor-Lopez, J., Puett, R., Rajagopalan, S., Brook, R.D. Ambient air pollution: an emerging risk factor for diabetes mellitus. Curr Diab Rep. 2015; 15: 603.
- Brook, R.D., Xu, X., Bard, R.L., Dvonch, J.T., Morishita, M., Kaciroti, N., et al. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ. 2013; 448: 66-71.
- Goettems-Fiorin, P.B., Grochanke, B.S., Baldissera, F.G., Dos Santos, A.B., Homem de Bittencourt, P.I., Jr., Ludwig, M.S., et al. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice: stress response and extracellular to intracellular HSP70 ratio analysis. J Physiol Biochem. 2016; 72: 643-656.
- Feng, S., Gao, D., Liao, F., Zhou, F., Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol Environ Saf. 2016; 128: 67-74.
- Guo, T., Cheng, X., Wei, J., Chen, S., Zhang, Y., Lin, S., et al. Unveiling causal connections: Long-term particulate matter exposure and type 2 diabetes mellitus mortality in Southern China. Ecotoxicol Environ Saf. 2024; 274: 116212.
- Hectors, T.L., Vanparys, C., van der Ven, K., Martens, G.A., Jorens, P.G., Van Gaal, L.F., et al. Environmental pollutants and type 2 diabetes: a review of mechanisms that can disrupt beta cell function. Diabetologia. 2011; 54: 1273-1290.
- Napolitano, T., Silvano, S., Ayachi, C., Plaisant, M., Sousa-Da-Veiga, A., Fofo, H., et al. Wnt Pathway in Pancreatic Development and Pathophysiology. Cells. 2023; 12.
- Chiang Yt A, Ip W, Jin T. The role of the Wnt signaling pathway in incretin hormone production and function [Review]. Frontiers in Physiology. 2012; 3: 273.
- Le Bacquer, O., Shu, L., Marchand, M., Neve, B., Paroni, F., Kerr Conte, J., et al. TCF7L2 splice variants have distinct effects on beta-cell turnover and function. Hum Mol Genet. 2011; 20: 1906-1915.
- Osmark, P., Hansson, O., Jonsson, A., Rönn, T., Groop, L., Renström, E. Unique splicing pattern of the TCF7L2 gene in human pancreatic islets. Diabetologia. 2009; 52: 850-854.
- Weise, A., Bruser, K., Elfert, S., Wallmen, B., Wittel, Y., Wöhrle, S., Hecht, A. Alternative splicing of Tcf7l2 transcripts generates protein variants with differential promoter-binding and transcriptional activation properties at Wnt/ beta-catenin targets. Nucleic Acids Res. 2010; 38: 1964-1981.
- Pang, D.X., Smith, A.J., Humphries, S.E. Functional analysis of TCF7L2 genetic variants associated with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2013; 23: 550-556.
- Shu, L., Matveyenko, A.V., Kerr-Conte, J., Cho, J.H., McIntosh, C.H., Maedler, K. Decreased TCF7L2 protein levels in type 2 diabetes mellitus correlate with downregulation of GIP- and GLP-1 receptors and impaired beta-cell function. Hum Mol Genet. 2009; 18: 2388-2399.
- Alfano R, Herceg Z, Nawrot TS, Chadeau-Hyam M, Ghantous A, Plusquin M. The Impact of Air Pollution on Our Epigenome: How Far Is the Evidence? (A Systematic Review). Curr Environ Health Rep. 2018; 5: 544-578.
- Shafiekhani, M., Ommati, M.M., Azarpira, N., Heidari, R., Salarian, A.A. Glycine supplementation mitigates lead-induced renal injury in mice. J Exp Pharmacol. 2019; 11: 15-22.
- Augereau C, Lemaigre FP, Jacquemin P. Extraction of high-quality RNA from pancreatic tissues for gene expression. Anal Biochem. 2016; 1: 60-62.
- Neuman, J.C., Truchan, N.A., Joseph, J.W., Kimple, M.E. A method for mouse pancreatic islet isolation and intracellular cAMP determination. J Vis Exp. 2014; e50374.
- Fraulob, J.C., Ogg-Diamantino, R., Fernandes-Santos, C., Aguila, M.B., Mandarim-de-Lacerda, C.A. A Mouse Model of Metabolic Syndrome: Insulin Resistance, Fatty Liver and Non-Alcoholic Fatty Pancreas Disease (NAFPD) in C57BL/6 Mice Fed a High Fat Diet. J Clin Biochem Nutr. 2010; 46: 212-223.
- Vasu, S., Moffett, R.C., Thorens, B., Flatt, P.R. Role of Endogenous GLP-1 and GIP in Beta Cell Compensatory Responses to Insulin Resistance and Cellular Stress. PLOS ONE. 2014; 9: e101005.
- Dastgheib, S., Irajie, C., Assaei, R., Koohpeima, F., Mokarram, P. Optimization of RNA extraction from rat pancreatic tissue. Iran J Med Sci. 2014; 39: 282- 288.
- Lee, S., Muniyappa, R., Yan, X., Chen, H., Yue, L.Q., Hong, E.G., et al. Comparison between surrogate indexes of insulin sensitivity and resistance and hyperinsulinemic euglycemic clamp estimates in mice. Am J Physiol Endocrinol Metab. 2008; 294: E261-270.
- van Dijk, T.H., Laskewitz, A.J., Grefhorst, A., Boer, T.S., Bloks, V.W., Kuipers, F., et al. A novel approach to monitor glucose metabolism using stable isotopically labelled glucose in longitudinal studies in mice. Lab Anim. 2013; 47: 79-88.
- Hu, Y., Shi, P., He, K., Zhu, Y.Q., Yang, F., Yang, M., et al. Methylation of Tcf712 promoter by high-fat diet impairs β-cell function in mouse pancreatic islets. Diabetes Metab Res Rev. 2018; 34: e2980.
- Yang, H., Li, Q., Lee, J.H., & Shu, Y. Reduction in Tcf7l2 Expression Decreases Diabetic Susceptibility in Mice [Research Paper]. International Journal of Biological Sciences. 2012; 8: 791-801.
- Shu, L., Zien, K., Gutjahr, G., Oberholzer, J., Pattou, F., Kerr-Conte, J., Maedler, K. TCF7L2 promotes beta cell regeneration in human and mouse pancreas. Diabetologia. 2012; 55: 3296-3307.
- Savic, D., Ye, H., Aneas, I., Park, S.Y., Bell, G. I., Nobrega, M.A. Alterations in TCF7L2 expression define its role as a key regulator of glucose metabolism. Genome Res. 2011; 21: 1417-1425.
- da Silva Xavier, G., Loder, M.K., McDonald, A., Tarasov, A.I., Carzaniga, R., Kronenberger, K., et al. TCF7L2 regulates late events in insulin secretion from pancreatic islet beta-cells. Diabetes. 2009; 58: 894-905.
- da Silva Xavier, G., Mondragon, A., Sun, G., Chen, L., McGinty, J.A., French, P.M., et al. Abnormal glucose tolerance and insulin secretion in pancreasspecific Tcf7l2-null mice. Diabetologia. 2012; 55: 2667-2676.
- Pradas-Juni, M., Nicod, N., Fernández-Rebollo, E.,Gomis, R. Differential transcriptional and posttranslational transcription factor 7-like regulation among nondiabetic individuals and type 2 diabetic patients. Mol Endocrinol. 2014; 28: 1558-1570.
- Kjems, L.L., Holst, J.J., Vølund, A., Madsbad, S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003; 52: 380-386.
- Nauck, M.A., Heimesaat, M.M., Orskov, C., Holst, J.J., Ebert, R., Creutzfeldt, W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993; 91: 301-307.
- Xu, G., Kaneto, H., Laybutt, D.R., Duvivier-Kali, V.F., Trivedi, N., Suzuma, K., King, G.L., et al. Downregulation of GLP-1 and GIP receptor expression by hyperglycemia: possible contribution to impaired incretin effects in diabetes. Diabetes .2007; 56: 1551-1558.
- Wang, F., Yoder, S.M., Yang, Q., Kohan, A.B., Kindel, T.L., Wang, J., et al. Chronic high-fat feeding increases GIP and GLP-1 secretion without altering body weight. Am J Physiol Gastrointest Liver Physiol .2015; 309: G807-815.
- Irwin, N., Flatt, P. Evidence for beneficial effects of compromised gastric inhibitory polypeptide action in obesity-related diabetes and possible therapeutic implications. Diabetologia. 2009; 52: 1724-1731.
- McClean, P.L., Irwin, N., Cassidy, R.S., Holst, J.J., Gault, V.A., Flatt, P.R. GIP receptor antagonism reverses obesity, insulin resistance, and associated metabolic disturbances induced in mice by prolonged consumption of high-fat diet. Am J Physiol Endocrinol Metab. 2007; 293: E1746-1755.
- Hansotia, T., Maida, A., Flock, G., Yamada, Y., Tsukiyama, K., Seino, Y., Drucker, D.J. Extrapancreatic incretin receptors modulate glucose homeostasis, body weight, and energy expenditure. J Clin Invest. 2007; 117: 143-152.
- Miyawaki, K., Yamada, Y., Ban, N., Ihara, Y., Tsukiyama, K., Zhou, H., et al. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nat Med. 2002; 8: 738-742.
- Kubo, F., Miyatsuka, T., Sasaki, S., Takahara, M., Yamamoto, Y., Shimo, N., et al. Sustained expression of GLP-1 receptor differentially modulates β-cell functions in diabetic and nondiabetic mice. Biochem Biophys Res Commun. 2002; 471: 68-74.
- Srivastava, S., Pandey, H., Tripathi, Y.B. Expression kinetics reveal the self-adaptive role of β cells during the progression of diabetes. Biomed Pharmacother. 2018; 106: 472-482.
- Aulinger, B.A., Vahl, T.P., Prigeon, R.L., D’Alessio, D.A., Elder, D.A. The incretin effect in obese adolescents with and without type 2 diabetes: impaired or intact? Am J Physiol Endocrinol Metab. 2016; 310: E774-781.
- Williams, L.M., Campbell, F.M., Drew, J.E., Koch, C., Hoggard, N., Rees, W.D., et al. The Development of Diet-Induced Obesity and Glucose Intolerance in C57Bl/6 Mice on a High-Fat Diet Consists of Distinct Phases. PLOS ONE. 2014; 9: e106159.
- Mosser, R.E., Maulis, M.F., Moullé, V.S., Dunn, J.C., Carboneau, B.A., Arasi, K., et al. High-fat diet-induced β-cell proliferation occurs prior to insulin resistance in C57Bl/6J male mice. Am J Physiol Endocrinol Metab. 2015; 308: E573-582.
- Xu, M.X., Ge, C.X., Qin, Y.T., Gu, T.T., Lou, D.S., Li, Q., et al. Prolonged PM2.5 exposure elevates risk of oxidative stress-driven nonalcoholic fatty liver disease by triggering increase of dyslipidemia. Free Radic Biol Med. 2019; 130: 542-556.
- Zheng, Z., Xu, X., Zhang, X., Wang, A., Zhang, C., Hüttemann, M., et al. Exposure to ambient particulate matter induces a NASH-like phenotype and impairs hepatic glucose metabolism in an animal model. J Hepatol. 2013; 58: 148-154.
- Miranda, R.A., da Silva Franco, C.C., Previate, C., Alves, V.S., Francisco, F.A., Moreira, V.M., et al. Particulate Matter Exposure During Perinatal Life Results in Impaired Glucose Metabolism in Adult Male Rat Offspring. Cell Physiol Biochem. 2018; 49: 395-405.
- Agnes Bosch, Christian Ott, Susanne Jung, Kristina Striepe, Marina V Karg, Dennis Kannenkeril, et al. How does empagliflozin improve arterial stiffness in patients with type 2 diabetes mellitus? Sub analysis of a clinical trial. Cardiovasc Diabetol. 2019; 18: 44.
- Gitto, S., Schepis, F., Andreone, P., Villa, E. Study of the Serum Metabolomic Profile in Nonalcoholic Fatty Liver Disease: Research and Clinical Perspectives. Metabolites. 2018; 8.
- Ballestri, S., Zona, S., Targher, G., Romagnoli, D., Baldelli, E., Nascimbeni, F., et al. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J Gastroenterol Hepatol. 2016; 31: 936-944.
- Mantovani, A., Byrne, C.D., Bonora, E., Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018; 41: 372-382.
- Chen SC, Tsai SP, Jhao JY, Jiang WK, Tsao CK, Chang LY. Liver Fat, Hepatic Enzymes, Alkaline Phosphatase and the Risk of Incident Type 2 Diabetes: A Prospective Study of 132,377 Adults. Sci Rep. 2017; 7: 4649.
- Meex, R.C.R., Watt, M.J. Hepatokines: linking nonalcoholic fatty liver disease and insulin resistance. Nat Rev Endocrinol. 2017; 13: 509-520.
- Xu, X., Yavar, Z., Verdin, M., Ying, Z., Mihai, G., Kampfrath, T., et al. Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol. 2010; 30: 2518-2527.
- Costa Beber, L.C., da Silva, M., Dos Santos, A.B., Mai, A.S., Goettems- Fiorin, P.B., Frizzo, M.N., et al. The association of subchronic exposure to low concentration of PM(2.5) and high-fat diet potentiates glucose intolerance development, by impairing adipose tissue antioxidant defense and eHSP72 levels. Environ Sci Pollut Res Int. 2010; 27: 32006-32016.