
Special Article - Current Endoscopic Therapy in Japan
Austin J Gastroenterol. 2015;2(2): 1035.
Endoscopic Mucosal Resection for Treatment of Multiple Early Gastric Cancer Complicating Familial Adenomatous Polyposis: A Case Report
Haruna Kawamura1*, Satohiro Matsumoto1, Hiroyuki Miyatani1, Yukio Yoshida1, Masaaki Saito2 and Yoh Dobashi3
1Department of Gastroenterology, Saitama Medical Center, Jichi Medical University, Japan
2Department of Surgery, Saitama Medical Center, Jichi Medical University, Japan
3Department of and Pathology, Saitama Medical Center, Jichi Medical University, Japan
*Corresponding author: Haruna Kawamura, Department of Gastroenterology, Saitama Medical Center, Jichi Medical University, 1-847 Amanuma, Omiya, Saitama, Saitama 330-8503, Japan
Received: December 19, 2014; Accepted: February 23, 2015; Published: February 25, 2015
Abstract
Familial Adenomatous Polyposis (FAP) is a hereditary colorectal disorder characterized by multiple adenomas developing diffusely throughout the large intestine that frequently show malignant transformation even at a young age. In a 69-year-old woman who had undergone total colectomy for FAP, endoscopic surveillance for multiple gastric polyps revealed a pedunculated polyp measuring more than 2 cm in diameter in the fornix of the stomach? As histopathological examination revealed that the polyp was an adenocarcinoma, Endoscopic Mucosal Resection (EMR) was performed to remove this polyp and three 1cm polyps around it. All the lesions were histopathologically diagnosed to be adenocarcinomas, and the risk of the remaining multiple gastric polyps progressing to cancer was also determined to be high. Therefore, total gastrectomy was performed. Histopathology of the total-gastrectomy specimen showed multiple intramucosal carcinomas. At present, there are no established guidelines for either surveillance or treatment of gastric lesions in FAP patients. As exemplified by our case, there are lesions among gastric polyps that are difficult to diagnose as cancer based on the endoscopic findings alone. For surveillance in FAP patients with multiple gastric polyps, step biopsy as well as target biopsy may need to be performed.
Keywords: Familial adenomatous polyposis; Gastric cancer; Endoscopic mucosal resection
Introduction
Familial Adenomatous Polyposis (FAP) is a peculiar hereditary colorectal disorder characterized by multiple adenomas developing diffusely throughout the large intestine that frequently show malignant transformation even at a young age. This disorder is considered to be caused by mutations of the adenomatous polyposis coli gene located on the long arm of chromosome 5 [1,2]. Meanwhile, in regard to extracolonic lesions associated with FAP, the disorder is known to be frequently complicated by gastric lesions [3,4]. Gastric lesions complicating FAP were first reported by Hauser [5], and several reports have been published since. The frequency of such lesions has also been reported to be quite high [4]. In a family study conducted by Watanabe et al, associated gastric lesions were detected in 15 patients from 13 families out of 22 patients from 14 families with FAP [6]. The gastric lesions known to be associated with FAP include fundic gland polyps [7,8], gastric adenomas [9], and gastric cancers [4], which are histologically specific to the fundic gland area. A recent study in 73 FAP patients with gastric lesions in Japan showed that the lesions comprised fundic gland polyps, gastric adenomas and gastric cancers, in that order of frequency, while the age at the time of detection was older in the reverse order [4].
Case Presentation
In a 69-year-old woman who had undergone total colectomy for FAP, endoscopic surveillance for multiple gastric polyps revealed a pedunculated polyp measuring more than 2 cm in diameter in the fornix of the stomach (Figure 1). Histopathology showed that the polyp was an adenocarcinoma; three subpedunculated polyps measuring about 1 cm in diameter around it were also suspected to be adenocarcinomas. Endoscopic Mucosal Resection (EMR) of all the four lesions was performed. All of the tissue specimens showed well-differentiated adenocarcinomas; furthermore, the polyp that measured more than 2 cm in diameter also showed sub mucosal invasion (Figure 2). Thus, the patient was diagnosed as having multiple gastric cancer, and it was suspected that cancer might also have developed in the remaining multiple polyps. Thus, after obtaining informed consent from the patient, total gastrectomy was additionally performed.
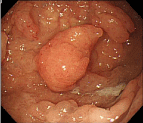
Figure 1: Upper gastrointestinal endoscopy. A pedunculated polyp measuring more than 2 cm in diameter was detected in the fornix of the stomach.
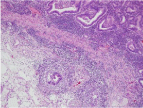
Figure 2: Tissue specimen obtained by EMR. The specimen was a welldifferentiated adenocarcinoma, showing sub mucosal invasion.
The total-gastrectomy specimen showed multiple polyps in the stomach extending from the cardiac to the body of the stomach. Although the majorities were benign lesions exhibiting fundic cystic polyp-like features, lesions with adenomatous atypia and cancer with prominent papillary and villous structures were found to be disseminated in the region from the upper body to the fornix of the stomach. All the cancers were confined to the mucosa. Lymph node metastasis was classified as pN1, and it was assumed that the metastasis might have arisen from the sub mucosal invasive cancer removed by EMR (Figure 3). The postoperative course was favorable, and the patient remains under follow-up to date.

Figure 3: Total gastrectomy specimen. Adenocarcinoma of the stomach, multiple, Tub1, Type 0-IIa, pM, ly0, v0, pPM0 (18mm), pDM0 (86mm), pN1, total gastrectomy. Green line: adenocarcinoma; Red line: adenomatous atypia.
Discussion
In Japan, the most common extracolonic lesion associated with FAP is gastric cancer. It has been reported that in Japan, 4.5%-13.6% of FAP patients have concomitant gastric cancer [10], and that the risk of development of gastric cancer is 3.44 times higher in patients with FAP [4]. In regard to the features of gastric cancer complicating FAP, there are many patients with multiple tumors; histologically, they are classified as well-differentiated adenocarcinoma in 60% of cases [10], and the most common site of occurrence is the gastric antrum [11,12]. However, the mechanism underlying malignant transformation of FAP is not yet clearly understood. Although fundic gland polyps have been frequently observed in FAP patients and been considered to be hyperplastic polyps with no potential for malignant transformation [13,14], there is one report of malignant transformation of fundic gland polyps complicating FAP [15].
The Spigelman classification [16] is used in the surveillance for duodenal adenoma in FAP patients; however, there are no clear guidelines for surveillance to detect gastric adenoma at present. Based on a report of detection of marked tumor growth in a patient undergoing annual surveillance [17], surveillance at shorter intervals needs to be considered.
Although the standard procedure for colonic adenoma complicating FAP is total colectomy, no established guidelines are currently available for the treatment of gastric adenoma [18]. Even in cases of early detection of gastric cancer, prophylactic total gastrectomy is not a standard procedure. In our patient, we were able to diagnose gastric cancer by performing biopsy of a largesized tumor. However, the cancers detected in the total-gastrectomy specimen could not be macroscopically differentiated from the noncancerous polyps. Moreover, the site of occurrence of gastric cancer in our case was not the pyloric antrum, which is considered to be a site of predilection, but the area around the fornix. Thus, for surveillance to detect gastric lesions in patients with FAP complicated by multiple gastric polyps, step biopsy as well as target biopsy may need to be performed.
Conclusion
As for the intervals and methods of surveillance for gastric lesions complicating FAP, further studies appear to be needed. Moreover, in cases of FAP complicated by multiple gastric cancers, total gastrectomy may need to be considered because of the limitations of endoscopic treatment.
References
- Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991; 66: 589-600.
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome sq21. Science. 1991; 253: 661-665.
- Utsunomiya J, Maki T, lwama T, Matsunaga Y, Ichikawa T. Gastric lesion of familial polyposis coli.Cancer. 1974; 34: 745-754.
- Iida M, Kobori Y, Mizuno M, Matumoto T. Extracolonic lesions associated with familial adenomatous polyposis. (in Japanese) Stomach and Intestine. 2000; 35: 327-336.
- Hauser G. Uber. Polyposis intestinalis adenomatosaund deren beziehungen zur Krbbsentwicklung. Arch F Kin Med. 1895; 55: 429-448.
- Watanabe H, Enjoji M, Yao T, Ohsato K. Gastric lesions in familial adenomatosis coli: their incidence and histologic analysis. Hum Pathol. 1978; 9: 269-283.
- Ranzi T, Castagnone D, Velio P, Bianchi P, Polli EE. Gastric and duodenal polyps in familial polyposis coli. Gut. 1981; 22: 363-367.
- Jagelman DG, DeCosse JJ, Bussey HJ. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988; 1: 1149-1151.
- Abraham SC, Montgomery EA, Singh VK, Yardley JH, Wu TT. Gastric adenomas: intestinal-type and gastric-type adenomas differ in the risk of adenocarcinoma and presence of background mucosal pathology. Am J Surg Pathol. 2002; 26: 1276-1285.
- Iwama T, Mishima Y, Utsunomiya J. The impact of familial adenomatous polyposis on the tumorigenesis and mortality at the several organs. Lts rational treatment. Ann Surg. 1993; 217: 101-108.
- Jagelman DG, DeCosse JJ, Bussey HJR. The Leeds Castle Polyposis Group. Upper gastrointestinal cancer in familial adenomatous polyposis. Lancet. 1988; 8595: 1149-1151.
- Watanabe H. Enjoji M, Yao T. Ohsato K. Gastric lesions in familial adenomatosis coli. Cancer. 1974; 34: 745-754.
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008; 6: 180-185.
- Teichmann J, Weickert U, Riemann JF. Gastric fundic gland polyps and colonic polyps: is there a link, really? Eur J Med Res. 2008; 13: 192-195.
- Garrean S, Hering J, Saied A, Jani J, Espat NJ. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg. 2008; 74: 79-83.
- Spigelman AD, Williams CB, Talbot IC, Domizio P, Phillips RK. Upper gastrointestinal cancer in patients with familial adenomatouspolyposis. Lancet. 1990; 2: 783-743.
- Osumi H, Ishiyama A, Hirasawa T, You T, Yamamoto Y, Tuchida T, et al. Early gastric adenocarcinoma arising from a gastric adenoma in a patient with familial adenomatous polyposis syndrome. (in Japanese) Progress of Digestive Endoscopy. 2014; 84: 108-110.
- Takeda A, Ban S, Tabuchi S, Aikawa K, Sinozuka N, Koyama I. Gastric lesions in familial adenomatous polyposis coli and gastric carcinogenesis. J Fam Tumor. 2007; 7: 30-35.