
Special Article - Current Endoscopic Therapy in Japan
Austin J Gastroenterol. 2015;2(2): 1036.
Salvage Endoscopic Treatment after Definitive Chemoradiotherapy for Esophageal Cancer
Shunsuke Urayoshi1*, Satohiro Matsumoto1, Hiroyuki Miyatani1, Yukio Yoshid1, Yoh Dobashi2 and Shigeki Yamada2
1Department of Gastroenterology, Saitama Medical Center, Jichi Medical University, Japan
2Department of Pathology, Saitama Medical Center, Jichi Medical University, Japan
*Corresponding author: Shunsuke Urayoshi, Department of Gastroenterology, Saitama Medical Center, Jichi Medical University, 1-847 Amanuma, Omiya, Saitama, Saitama 330-8503, Japan
Received: December 19, 2014; Accepted: February 23, 2015; Published: February 25, 2015
Abstract
We describe our experience with 2 patients with esophageal cancer who underwent endoscopic treatment as salvage treatment after Chemo Radio Therapy (CRT). A 60-year-old man who received CRT for esophageal cancer (T2N0M0, stage II) developed local recurrence of the cancer 1 year and 4 months after the completion of CRT and underwent Endoscopic Submucosal Dissection (ESD) as salvage treatment. He did not develop any recurrence for 3 years after the ESD, but eventually died of intercurrent disease. A 69-yearold man who received CRT for pharyngeal cancer (T4N2M0, stage IV) and early esophageal cancer (TlbN0M0, stage I) developed local recurrence of the esophageal cancer 6 months after the completion of CRT and underwent Endoscopic Mucosal Resection (EMR) as salvage treatment. He remains alive without recurrence 5 years after the EMR. Although the indications for salvage endoscopic treatment need to be carefully examined, this treatment appears to be useful for local recurrence of esophageal cancer developing after CRT.
Keywords: Esophageal cancer; Salvage treatment; Endoscopic submucosal dissection; Endoscopic mucosal resection; Chemoradiotherapy
Introduction
Treatments for superficial esophageal cancer include endoscopic treatment, surgery, and Chemo Radio Therapy (CRT). In the JCOG9708 Study, the complete response rate of stage I esophageal cancer patients to definitive CRT was 87.5% and the 4-year overall survival rate was 80.5%, the treatment efficacy being comparable to surgical treatment [1]. Meanwhile, recurrence following CRT reportedly occurs in approximately 45% of the patients, and most of these are local recurrences [2]. In recent years, salvage endoscopic treatment has been performed following early diagnosis of local recurrence; it has been reported to be favorable in terms of both the safety and long-term outcomes [3-5]. However, no clear consensus has been reached regarding the indications of salvage endoscopic treatment, methods of surveillance following endoscopic treatment, and the testing intervals. We describe our experience with 2 patients with esophageal cancer who developed local recurrence after definitive CRT, in whom local control was achieved by salvage endoscopic treatment.
Case Presentation
Case 1
60-year-old man who underwent endoscopy during a routine health check-up was incidentally diagnosed as having stage II (advanced) cancer (T2N0M0) of the middle esophagus (Figure 1). The patient presented with alcoholic liver cirrhosis and hepatic dysfunction, with an indocyanine green retention rate at 15 minutes of 15.3%, and received CRT with combined CDDP and 5-FU regimen. The tumor disappeared and the patient was judged as having shown Complete Response (CR) to the treatment. Endoscopic examination, performed at 1 year and 4 months after the CRT, showed a depressed lesion measuring 10 mm in diameter at the same site, iodine staining showed an irregular unstained area, and biopsy revealed the features of Squamous Cell Carcinoma (SCC). The patient was diagnosed as having cancer recurrence (Figure 2). Based on the endoscopic findings, the lesion was determined as an intramucosal carcinoma based on the depth of invasion, and absence of evidence, on computed tomography, of distant metastasis or lymph node metastasis. We obtained informed consent from the patient and performed Endoscopic Submucosal Dissection (ESD) as a salvage treatment. Although the procedure was completed without complications, severe fibrosis of the submucosa made the detachment procedure difficult. The histopathological diagnosis was deep submucosal infiltration, unknown status of the surgical margin, and no vascular invasion (Figure 3). Since it became impossible to determine the status of the surgical margin due to the influence of cauterization, the possibility of local recurrence was explained to the patient. However, he did not wish to undergo additional surgery and elected to receive endoscopic surveillance every 3 months. No evidence of recurrence was detected for a period of 1 year and endoscopic surveillance was carried out at an interval of 6 to 12 months thereafter. Despite not showing any evidence of recurrence, the patient developed hepatocellular carcinoma 3 years after the ESD and died.
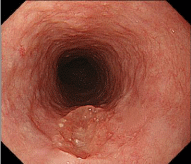
Figure 1: Endoscopic findings of the upper esophagus. The middle esophagus showed a depressed lesion measuring 15 mm in diameter, with an irregular depressed surface, showing little change in the form of the lesion after air insufflation and disinflation.
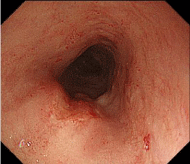
Figure 2: Endoscopic findings of the upper esophagus. The middle
esophagus showed a depressed lesion measuring 10 mm in diameter. Despite
manifesting as an irregular unstained area on iodine staining, the lesion was
not high but flat, and was determined to be intramucosal carcinoma based on
the invasion depth.
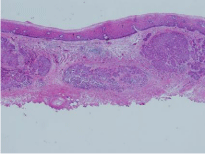
Figure 3: Histopathological specimen of endoscopic submucosal dissection. Submucosal deep infiltration is observed.
Case 2
A 69-year-old man underwent detailed endoscopic examination for a strange sensation in the pharynx and was diagnosed as having double cancers: pharyngeal cancer (T4N2M0, stage IV) and early esophageal cancer (TlbN0M0, stage I) (Figure 4). He was administered two cycles of chemotherapy with CDDP and 5-FU, followed by concurrent CRT with carboplatin. Both the pharyngeal cancer and the esophageal cancer disappeared, and the patient was judged as showing CR. However, endoscopic examination, performed 6 months after the CRT, showed a flat elevated lesion measuring 2 mm in diameter in the middle esophagus, and biopsy revealed the features of SCC, based on which the patient was diagnosed as having recurrent cancer (Figure 5a). Since endoscopic ultrasonography showed that the submucosal layer was intact, it was determined as an intramucosal lesion (Figure 5b). After obtaining informed consent from the patient, EMR was performed as a salvage therapy. Histopathological examination revealed invasion of the muscularis mucosae, a negative surgical margin, and no vascular invasion (Figure 6). The patient remains alive without any evidence of recurrence 5 years after the EMR.
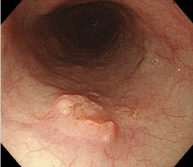
Figure 4: Endoscopic findings of the upper esophagus. The middle esophagus showed a flat elevated lesion measuring 15 mm in diameter with slightly distinct raised surroundings. The invasion depth was determined as the submucosa.
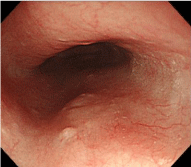
Figure 5a: Endoscopic findings of the upper esophagus. A depressed lesion measuring 2 mm in diameter was observed in the middle esophagus.
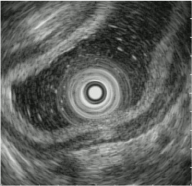
Figure 5b: Endoscopic ultrasound findings. As the submucosal layer was intact, the lesion was determined as intramucosal lesion.
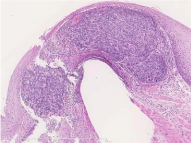
Figure 6: Histopathological specimen of endoscopic mucosal resection. Invasion of the muscularis mucosae.
Discussion
The indication of definitive CRT for esophageal cancer has been expanded to patients who cannot undergo resection, in addition to those who are suitable candidates for surgical resection; the treatment has been shown to yield results comparable to those of surgical resection [1]. However, we occasionally encounter patients with remnant and recurrent carcinoma at the primary site, with the reported rate of recurrence being as high as approximately 45% [6]. A current issue in definitive CRT is that there are no clear guidelines for second-line treatment of patients with remnant or recurrent cancer. No chemotherapy has been shown to yield favorable results as salvage treatment, and surgery is selected, in general. The 5-year survival rate of patients undergoing salvage surgical treatment has been reported to be approximately 25% [7,8]; however, due to the high invasiveness and high hospital mortality, a less invasive treatment has been sought. In recent years, salvage endoscopic therapy has been shown to be effective against remnant and recurrent esophageal cancer at the primary site following CRT, and it is considered to be a radical treatment, reportedly yielding a 5-year survival rate of 49.1% [9]. The recurrence rate in lymph nodes in patients showing CR following CRT is very low, being around 1% [10]. That is, salvage therapy effective against local recurrence is highly likely to result in a prolongation of the survival period in patients with esophageal cancer who develop local recurrence after showing CR to CRT. Both of our patients remained recurrence-free for a long period following the endoscopic treatment.
Next, there is the issue of the diagnosis of local recurrence after definitive CRT. As CRT affects the properties and progression form of the cancer, endoscopic diagnosis of a local recurrence after CRT should not be dealt with in the same manner as usual early esophageal cancer. A report based on detailed examination of pathological specimens after CRT showed that the form of recurrence differed between patients showing and those not CR to CRT; tumor cells remain in all the layers in patients who do not show CR, whereas normal mucous membrane covers the surface in patients achieving CR. Thus, tumor cells remain in deep layers such as the muscular layer and outer membrane [11]. This lends support to the report of Muto et al. that endoscopic examination in patients developing local recurrence after achieving CR to CRT frequently shows submucosal tumor-like findings [12]. For the evaluation of local recurrence developing after definitive CRT, endoscopic ultrasonography is expected to be beneficial in addition to magnifying endoscopy using narrow-band imaging and iodine staining diagnosis.
In addition, no clear consensus has been reached regarding the appropriate period of surveillance. The guidelines in Japan state that surveillance should be carried out every 3 months during the first year after the completion of CRT and every 4 to 6 months thereafter [13]; however, the basis and rationale for the use of this method are not clear. Individual differences in the responses to treatment are a factor that makes it difficult to determine the period of efficacy maintenance and the onset of efficacy after the start of CRT. Ishihara et al. reported that the recurrence predictors in 110 patients who achieved CR with CRT included the T factor for local recurrence, and the age and primary tumor site for lymph node metastasis and distant metastasis [14]. It would be desirable for a surveillance method tailored to individual patients, with stratified recurrence risks following CRT, to be established in the future.
Conclusion
We describe our experience with twp patients with esophageal cancer who developed local recurrence after definitive CRT, in whom local control could be achieved by endoscopic treatment.
References
- Kato H, Sato A, Fukuda H, Kagami Y, Udagawa H, Togo A, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol. 2009; 39: 638-643.
- Gardner-Thorpe J, Hardwick RH, Dwerryhouse SJ. Salvage oesophagectomy after local failure of definitive chemoradiotherapy. Br J Surg. 2007; 94: 1059-1066.
- Ishii N, Suzuki K, Fujita Y. Salvage endoscopic submucosal dissection for recurrent esophageal squamous-cell carcinoma after definitive chemoradiotherapy. Clin J Gastroenterol. 2011; 4: 85-88.
- Saito Y, Takisawa H, Suzuki H, Takizawa K, Yokoi C, Nonaka S, et al. Endoscopic submucosal dissection of recurrent or residual superficial esophageal cancer after chemoradiotherapy. Gastrointest Endosc. 2008; 67: 355-359.
- Hattori S, Muto M, Ohtsu A, Boku N, Manabe T, Doi T, et al. EMR as salvage treatment for patients with locoregional failure of definitive chemoradiotherapy for esophageal cancer. Gastrointest. Endosc. 2003; 58: 65-70.
- Ohtsu A. Chemoradiotherapy for esophageal cancer: current status and perspectives. Int J Clin Oncol. 2004; 9: 444-450.
- Swisher SD, Wynn P, Putnam JB, Mosheim MB, Correa AM, Komaki RR, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg. 2002; 123: 175-183.
- Chao YK, Chan SC, Chang HK, Liu YH, Wu YC, Hsieh MJ, et al. Salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur J Surg Oncol. 2009; 35: 289-294.
- Yano T, Muto M, Hattori S, Minashi K, Onozawa M, Nihei K, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy. 2008; 40: 717-721.
- Onozawa M, Nihei K, Ishikura S, Minashi K, Yano T, Muto M, et al. Elective nodal irradiation in definitive chemoradiotherapy for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol. 2009; 92: 266-269.
- Iwama M, Yasuda T, Konno M, Nakamori Y, Nishiyama A, Takemoto T, et al. Clinical and pathological studies on remnant or recurrent esophageal cancer after definitive chemoradiotherapy. (in Japanese) Med J Kinki Univ. 2009; 34 : 113-122.
- Tu CH, Muto M, Horimatsu T, Taku K, Yano T, Minashi K, et al. Submucosal tumor appearance is a useful endoscopic predictor of early primary-site recurrence after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Dis. Esophagus. 2011; 24: 274-278.
- Japanese Society for Esophageal Disease. Guidelines for diagnosis and treatment on cartinoma of the esophagus. 3rd ed. : Kanehara- shuppan. 2012.
- Ishihara R, Yamamoto S, Iishi H, Takeuchi Y, Sugimoto N, Higashino K, et al. Factors predictive of tumor recurrence and survival after initial complete response of esophageal squamous cell carcinoma to definitive chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010; 76: 123-129.