
Case Report
Austin J Gastroenterol. 2015;2(3): 1042.
Endoscopic Mucosal Resection for Early Gastric Cancer Arising in a Small Adenomatous Polyp
Gerada J1*, Fenech V1, Attard J2 and Pocock J1
1Division of Gastroenterology, Mater Dei Hospital, Malta
2Department of Pathology, Mater Dei Hospital, Malta
*Corresponding author: Dr. Jurgen Gerada MD MRCP MSc, Mater Dei Hospital, Msida, Malta, Europe
Received: December 07, 2014; Accepted: March 09, 2015; Published: March 11, 2015
Keywords
Adenoma; Endoscopic Mucosal Resection; Gastric Cancer; Polyp
Introduction
Gastric cancer is the sixth commonest cancer and the fourth commonest cause of cancer-related deaths. Despite a gradual decline in the worldwide incidence of gastric cancer, there has been a relative increase in the incidence of tumors of the oesophago-gastric junction and gastric cardia. Advances in technology has made it possible for improvements in diagnostic and therapeutic endoscopy, and staging with cross-sectional imaging. Moreover, the increase in the elderly population with multiple co-morbidities is presenting significant clinical management challenges.
We hereby present an elderly lady, who was found to have early gastric carcinoma in the antrum of the stomach, arising from a small adenomatous polyp and treated with Endoscopic Mucosal Resection (EMR). This case highlights the successful endoscopic treatment with EMR in early gastric cancer, especially in patients who would not be fit for surgery. It also highlights the progression of gastric adenomas to carcinoma even in small polyps, when classically this is associated with larger polyps.
Case Presentation
An 87 year old lady, with a history of coronary artery bypass graft, ischaemic heart disease, hypertension and chronic renal failure, presented with a 3 day history of melaena and subsequent anaemiarelated decompensated congestive heart failure. She did not report any other associated upper or lower gastrointestinal symptoms. She had been taking dual antiplatelet therapy and a once daily proton pump inhibitor, amongst other medications for hypertension. Family and social histories were unremarkable. Clinical examination revealed pallor, tachycardia and hypotension, but failed to reveal any abdominal masses or scars. Digital rectal examination confirmed melaena. Blood investigations showed a normochromic normocytic anaemia (haemoglobin 7.2g/dL (11.5-16.5g/dL)), a low normal ferritin of 22ng/mL (range 10-291ng/mL), iron of 7.15umol/L (range 5.8-34.5umol/L) and transferrin saturation of 11% (20-50%). Her haemoglobin had been normal 3 months previously. Following discontinuation of antiplatelet therapy, administration of blood products and resuscitation of the patient, an upper Gastrointestinal Endoscopy (OGD) was performed. This showed a small (6mm), lobulated, sessile, ulcerated antral polyp, with surrounding erythema and few superficial types of erosion (Figure 1). Histology showed the polyp to be adenomatous with low grade dysplasia with foci suspicious for malignancy. Surrounding antral mucosa showed congested lamina propria, prominent foveolar hyperplasia and intestinal metaplasia. No Helicobacter pylori organisms could be seen. A computed tomography of the trunk revealed no evidence of metastasis. Endoscopic ultrasound could not be possible, as this is unavailable in our centre.
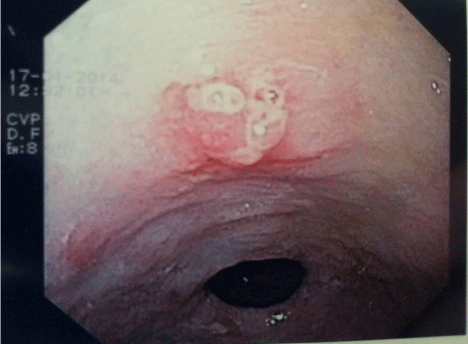
Figure 1: Small ulcerated lobulated antral polyp harbouring gastric malignant
cells.
Given the general unfitness of the patient for any surgical interventions due to her comorbidities, the option of Endoscopic Mucosal Resection (EMR) was pursued. At a repeat OGD, the polyp was lifted with adrenaline and saline and hot snare polypectomy was carried out en bloc. A clip was applied to the base of the resected area to reduce the risk of post-polypectomy bleeding. Histology of the resected polyp showed a moderately well differentiated adenocarcinoma arising in an adenomatous polyp (Figure 2). Nonneoplastic mucosa showed chronic gastritis with intestinal metaplasia. The patient remains well and asymptomatic 8 months following the procedure, without any evidence of local recurrence or disseminated disease.
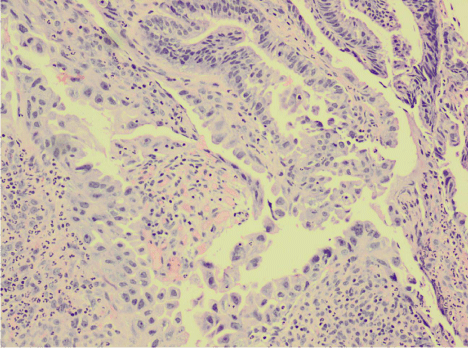
Figure 2: Resected antral polyp showing adenocarcinoma cells (H&E, x100).
Discussion/Conclusion
Most gastric polyps are found incidentally at endoscopy and are usually asymptomatic, especially if they are small. Histological diagnosis of gastric polyps is essential to assess their malignant potential. All gastric polyps except fundic gland polyps and inflammatory fibroid polyps are associated with significantly increased gastric cancer risk, especially adenomatous and hyperplastic polyps. Clinical indications for complete removal of gastric polyps include polyps larger than 1cm, those with dysplastic foci and symptomatic polyps such as bleeding, abdominal pain or gastric outlet obstruction [1].
Adenomatous polyps account for approximately 10% of gastric polyps and have significant malignant potential, as well as its surrounding mucosa. Histologically, they can be classified as tubular, villous or tubulovillous, and are commonly found in the antrum. They are frequently solitary and arise on a background of atrophic gastritis and intestinal metaplasia [2]. A malignant focus is detected in less than 10% of cases but is more frequent when the lesion is larger than 2 cm, villous in nature and occurring in the elderly [2]. Guidelines have therefore recommended that adenomatous polyps are completely removed when safe to do so, and biopsies taken from the surrounding mucosa or any visible mucosal abnormalities. Following complete polypectomy, in the absence of high grade dysplasia, a repeat gastroscopy is suggested after one year [1].
The sequence of events for the development of gastric carcinoma, proposed by Correa et al in 1988, had shown that the intestinaltype gastric cancer sequentially transformed from normal mucosa into atrophic gastritis, intestinal metaplasia, dysplasia and finally to invasive adenocarcinoma (Metaplasia-Adenoma-Carcinoma Sequence (MACS)), in a similar fashion to the multi-step sequence of events in colorectal cancer [3]. This was subsequently supported by extensive studies by Keller et al in 2005 [4]. Helicobacter pylorus was not shown to be linked with such adenomas [1]. Although various studies reported such sequence, its exact mechanism is yet to be fully characterized. P53 was shown to play a less crucial part in gastric MACS than in the colon, while Ki-ras gene mutations were not detected in such lesions, suggesting that the MACS in the stomach has a different mechanism from that in the colon [5]. High levels of Cyclo-Oxygenase-2 (COX-2) in the mucosa has been linked to gastric cancer progression [6], while tissue factor has also been involved in tumor progression along the MACS of gastric cancer [7]. Further research is now being directed to the potential use of COX-2 inhibitor like aspirin as chemoprevention for gastric cancer [6]. While nonmolecular markers such as polyp size >2cm, villous histology and older age seem to be greater risk factors for neoplastic progression, in the case of our patient, malignant transformation occurred in a polyp of 6mm, which is an unusual presentation for gastric malignancy.
Endoscopic therapy has an increasing role in the management of dysplasia and early gastric cancers. With the introduction of EMR and Endoscopic Submucosal Resection (ESD) for early gastric cancer, longterm survival can be achieved [8], thus avoiding gastrectomy. In fact these endoscopic treatments have now been included in international guidelines, as part of the therapeutic options in early gastric cancer [9]. Following the success of these endoscopic treatments, the roles of EMR and ESD have been extended by the Japanese Gastric Cancer Association from mucosal cancer, well differentiated non-ulcerated small lesion (<2 cm), to any size (elevated), ulcerated (<3 cm) and includes undifferentiated subtype [10]. EMR (R0) resection has been shown to be safe in the elderly [11] and results are comparable to gastrectomy in terms of risk of death and local recurrence [12] (6% after a median of 17.9 months [13]), but with shorter hospital stay [14]. In a study by Nakamoto S et al, it was found that EMR was comparable to ESD for early gastric cancer of 5mm or less, and suggested that such lesions should be treated with EMR [15]. Given the age and comorbidities of our patient, and the small tumor size, it was decided that EMR would be the initial treatment proposed. No evidence of recurrence has been noted during follow-up following EMR.
This case highlights the importance of multiple biopsies even in small lesions which look suspicious, as well as its surrounding mucosa. It emphasizes that early gastric cancer, although classically arises in large lesions, can also occur in small lesions. This case also confirms the underlying pathological metaplasia-adenomacarcinoma sequence in gastric cancer. Finally, it supports the fact that EMR is a good therapeutic option in early gastric cancer and allows less invasive treatment without sacrificing the possibility of cure, especially in small lesions and in the elderly with many comorbidities.
References
- Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B. British Society of Gastroenterology. The management of gastric polyps. Gut. 2010; 59: 1270-1276.
- Islam RS, Patel NC, Lam-Himlin D, Nguyen CC. Gastric polyps: a review of clinical, endoscopic, and histopathologic features and management decisions. Gastroenterol Hepatol (NY). 2013; 9: 640-651.
- Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988; 48: 3554-3560.
- Keller G, Hofler H, Becker KF. Molecular medicine of gastric adenocarcinomas. Expert Rev Mol Med. 2005; 7: 1-13.
- Sakurai S, Sano T, Maeshima A, Kashiwabara K, Oyama T, Fukuda T, et al. Gastric adenoma-carcinoma sequence with special reference to p53 and Ki-ras gene alterations. Virchows Arch. 1995; 427: 119-124.
- Wang Z, Chen JQ, Liu JL. COX-2 Inhibitors and Gastric Cancer. Gastroenterol Res Pract. 2014; 132320.
- Lo L, Valentine H, Harrison J, Hayes S, Welch I, Pritchard S, et al. Tissue factor expression in the metaplasis-adenoma-carcinoma sequence of gastric cancer in a European population. Br J Cancer. 2012; 107: 1125-1130.
- Uedo N, Iishi H, Tatsuta M, Ishihara R, Higashino K, Takeuchi Y, et al. Longterm outcomes after endoscopic mucosal resection for early gastric cancer. Gastric Cancer. 2006; 9: 88-92.
- Allum WH, Blazeby JM, Griffin SM, Cunningham D, Jankowski JA, Wong R. Association of Upper Gastrointestinal Surgeons of Great Britain and Ireland, the British Society of Gastroenterology and the British Association of Surgical Oncology. Guidelines for the management of oesophageal and gastric cancer. Gut. 2011; 60: 1449-1472.
- Sugano K. Gastric cancer: pathogenesis, screening, and treatment. Gastrointest Endosc Clin N Am. 2008; 18: 513-522.
- Etoh T, Katai H, Fukagawa T, Sano T, Oda I, Gotoda T, et al. Treatment of early gastric cancer in the elderly patient: results of EMR and gastrectomy at a national referral center in Japan. Gastrointest Endosc. 2005; 62: 868-871.
- Ono H, Kondo H, Gotoda T, Shirao K, Yamaguchi H, Saito D, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut. 2001; 48: 225-229.
- Kim JJ, Lee JH, Jung HY, Lee GH, Cho JY, Ryu CB, et al. EMR for early gastric cancer in Korea: a multicenter retrospective study. Gastrointest Endosc. 2007; 66: 693-700.
- Choi KS, Jung HY, Choi KD, Lee GH, Song HJ, Kim do H, et al. EMR versus gastrectomy for intramucosal gastric cancer: comparison of long-term outcomes. Gastrointest Endosc. 2011; 73: 942-948.
- Nakamoto S, Sakai Y, Kasanuki J, Kondo F, Ooka Y, Kato K, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy. 2009; 41: 746-750.