
Research Article
Austin J Gastroenterol. 2015;2(4): 1047.
Lower Gastrointestinal Tract Functional Disorders: Prevalence and Symptoms Characteristics in Outpatient Gastroenterology Clinic
Mahamane Sani LA¹*, Liu Jinsong¹ and Mahaman Yacoubou AR²
¹Department of Gastroenterology, Huazhong University of Science and Technology, China
²Department of Pathology and Pathophysiology, Huazhong University of Science and Technology, China
*Corresponding author: Mahamane Sani LA, Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (HUST) Wuhan/China, 13 Hang Kong Road, Wuhan, Hubei, P.R. China
Received: March 24, 2015; Accepted: April 23, 2015; Published: April 30, 2015
Abstract
Background: Worldwide digestive diseases are common in population. Functional Gastrointestinal Disorders (FGIDs) consist of a collection of chronic or recurrent symptoms attributed to the gastrointestinal tract that can range from esophagus to rectum and cannot be explained by structural or biochemical abnormalities. FGIDs are defined essentially by symptoms association and almost few limited tests are required to provide their diagnosis.
Objectives: The principal objective of this study was to evaluate the prevalence of FGIDs (Functional Abdominal Pain and Functional Bowel Disorders) and to investigate the possible associations between age, sex, psychological factors, drugs intake and FGIDs.
Method: Through a cross sectional study, a total of 1002 symptomatic patients without previous diagnosis of disease in who after consulting in outpatient clinic were prescribed colonoscopy completed a validated questionnaire. FGIDs were diagnosed according to Rome III diagnostic criteria.
Results: The mean age was 43.72 years, 55% (552) of subjects were males and 45% (450) females. The prevalence of overall Functional GI disorders was 55.7% and that by specific FGID was as follows: IBS 24.95%, functional constipation 22.75 %, functional diarrhoea 21.05 %, functional bloating 28.94%, unspecified functional bowel disorder 11.87% and functional abdominal pain 24.75%. Around 10% of subjects are “unclassified patients”. The overlapping syndrome among FGIDs (multiple FGIDs) is high and represents 72.04% with patients having 2 coexisting FGID 29.39%, 3 coexisting FGID 25.04% and more than 3 coexisting FGID 17.56%. Subjects having history of psychological event and drugs intake represent 51.5% and 16.37% respectively.
Conclusion: FGIDs were common in this study, as do their overlapping what deserve greater attention. There is influence of age, gender, psychological factors and drugs intake on FGIDs occurrence and symptoms modulations.
Keywords: Prevalence; LGITFDs; Colonoscopy; Rome III criteria; Psychological factors, Drugs intake
Abbreviations
FB: Functional Bloating; FD: Functional Diarrhea; FC: Functional Constipation; FUBD: Functional Unspecified Bowel Disorder; FAP: Functional Abdominal Pain; FBD/FBDs: Functional Bowel Disorder/ Functional Bowel Disorders; FGID/FGIDs: Functional Gastrointestinal Disorder/ Functional Gastrointestinal Disorders; IBS: Irritable Bowel Syndrome; GI: Gastrointestinal; LGIT: Lower Gastrointestinal Tract; LGITFDs: Lower Gastrointestinal Tract Functional Disorders
Introduction
Worldwide digestive diseases are common in population. Digestion is a complex process from mouth to anus, combining anatomic, mechanical, hormonal, enzymatic, neurologic factors. Although multiple factors affect the food behaviour: ethnicity, geography, environment, race, but the most important are availability, hygiene and quality of food in order to obtain a well balanced diet. Digestive disorders can range from mild to severe and from acute to chronic. They can be accompanied with pain or not in one hand and benign or malignant in the other hand.
Functional Gastrointestinal Disorders (FGIDs) consist of a collection of chronic or recurrent symptoms attributed to the gastrointestinal tract that can range from oesophagus to rectum and cannot be explained by structural or biochemical abnormalities [1]. These symptoms develop from abnormalities in gastrointestinal functionality which could be motility, increased nerve sensitivity of the intestinal tract or dysregulation of the brain-gut nerve pathways. Symptoms produced can be any combination of: nausea, vomiting, heartburn chest, abdominal or rectal pain or discomfort, diarrhoea, or constipation. When these GI symptoms persist for a certain period of time (3 months, 6 months, 1 year) according to specific diagnostic criteria of a functional GI disorder (Manning, Kruis, or Rome I, II, III) and in the absence of alarming symptoms and organic lesions, they are diagnosed as a FGID. FGIDs are defined essentially by symptoms association and almost few limited tests are required to provide their diagnosis. Functional disorders had existed long ago in the populations, but not diagnosed at that time because of lack of sensitive means of diagnostic evaluations. The increasing progress in medical science especially in Imagery (CT, Ultrasound, Endoscopy, MRI, ERCP, etc.) and Histochemestry with development of biological markers for tumour detection as well as in Pathology, Biology, and Biochemistry have improved and increased the diagnosis in Gastroenterology’s domain. So, after exhaustion of all means of diagnostic without any obvious evidence of disease or lesion with the persistence of patient’s symptoms we could consequently sustain the diagnosis of FGID in contrast to organic disease. It is of great importance to precise that nowadays FGIDs are recognized as independent entities in gastroenterology clinic, so the classical opposition of functional to organic is misleading as it is limiting the understanding of this vast domain.
The GI functional disorders are gaining magnitude due to drastic changes of living conditions and diet habits (alimentary industry, large pesticides using, expansion of GMOs food in the base diet). The link between food intake and symptom induction is recognized [2]. Also, hygiene of life is decreasing in population because of inactivity, obesity, tobacco, alcohol, flavourings and industrial colorant abuse, over-the-counter drugs abuse. This phenomenon plays an important role in digestive health deterioration.
Otherwise, the current development in gastroenterology science accompanied with more availability of gastroenterologists, new tools and techniques for gastrointestinal disease diagnosis should also be considered in the increased rate of FGID since it allows more investigative studies and improves diagnostic accuracy [3].
Additionally, FGIDs are gaining interest worldwide and this through the increase of related scientific publications, and the sensitization by media and internet [4].
FGIDs are highly prevalent disorders; indeed, up to 35% of the world population suffers from FGIDs accounting for about 40% of gastroenterology consultations and 12% of primary care practice [5]. However, FGIDs vary depending on the type of symptom and for the most common the median prevalence was 11% for IBS, 13.4 % for FD, above 15% for constipation worldwide [6], but also according to countries, geographic locations, sociocultural and sociodemographic features. For instance, prevalence rates were 55.24 % in china [7], 61.7% in Canada [8], 33% in Australia [9].
Although several epidemiologic studies have been conducted around the world, of note is the large disparities in the prevalence and incidence of FGIDs. More, epidemiologic knowledge is paramount and mandatory before leading off any disease diagnosis in clinical practice. Based on this observation, in this study we will address two (2) major categories among the FGIDs according to Rome III classification: Functional Bowel Disorders (FBDs) and Functional Abdominal Pain (FAP).
Methods
Type of study
It is a cross-sectional prospective study about 1002 observations using a self administered questionnaire and colonoscopy findings record during a period of 4 months in the Endoscopic Centre 1 of Union Hospital in Wuhan/China.
Inclusion criteria: patient undergoing colonoscopy in Endoscopic Centre 1 without any organic diseases diagnosis, willing to participate voluntarily.
Exclusion criteria were:
1. Normal colonoscopy findings that do not fulfill Rome III criteria (=“unclassified patients”)
2. Having an organic or structural disease diagnostic
3. Colonoscopy incomplete examination
Sampling
Randomly selected 1027 patients of all ages and sex who were admitted for colonoscopy at the endoscopic center 1 of outpatient gastroenterology clinic in Union Hospital, a university hospital of Huazhong University of Science and Technology (Wuhan) from July to October 2014 were recruited in the study before undergoing their examination. All patients complained of GI symptoms for a certain period of time and all were referred by a gastroenterologist for diagnostic colonoscopy after a consultation. Out of the 1027 respondents we obtain 1002 valid questionnaires for the study. The 25 questionnaires were removed because they did not complete their colonoscopy. FGIDs are defined by the presence of GI symptoms for at least 3 days per month in the last 3 months with symptom onset of at least 6 months before diagnosis. Then coupled a colonoscopic examination, minimal blood testing (CBC, ESR, CRP, fecal occult blood and calprotectin tests, and thyroid function) and presence or not of alarm symptoms in their diagnostic work-up.
All the patients have an educational background that allows them to complete the modified Rome III Chinese questionnaire. After explaining the study scope, a formal consent of patients was obtained before they get enrolled in the study, then patients’ anonymity was preserved. Approval of the ethic committee of Union Hospital was obtained for the present study.
Questionnaire
Three (3) different forms of the questionnaire have been tested in a small sample initially until we obtain the validated questionnaire for the study. A questionnaire in Chinese was designed and validated for the present study. The questionnaire includes multiple sets of questions, and 3 of them were designed to assess FGIDs according to the Rome III criteria. The functional disorders identified by the questionnaire included IBS, functional abdominal pain, functional abdominal bloating, functional diarrhea, functional constipation and unspecified functional bowel disorder and a FGID is defined as having FBDs, FAP or both. The others questions included were: demographic information (name, age, and sex), drugs intake history, psychological history, chief complaint, stools form and alarm symptoms.
The patients answered the question by themselves or if necessary with the assistance of a trained doctor or assistant. The completion of the questionnaire took an average of 15 minutes. When questions are misunderstood the interviewer explains and helps to confirm the answer. Patients were also helped with Bristol stools scale large pictures to identify their stools form.Then all respondents colonoscopy findings were recorded in the questionnaire later. Those with individual bowel symptoms unaccompanied by other symptoms that fulfilled the criteria for a syndrome were classified as unspecified functional bowel disorder.
Colonoscopy
Normal colonoscopy findings is defined when the total colon was checked and no lesions was found. The lesions that defined organic disease are classified as follows: hemorrhoids, polyps, colorectal cancer, colitis, diverticulosis, UC, CD, melanosis coli, ileitis, erythema and erosions, miscellaneous, colon varices, active bleeding, proctitis, and sigmoiditis. Incomplete colonoscopy is defined as a partial examination of colon.
Data analysis
Statistical analyses were carried out using SPSS software version 20.0 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation, and categorical data were presented as numbers and percentages in descriptive statistics, and 95% as CI. The difference and relationship between variables were evaluated using chi square, correlation and regression tests. A drown P = 0.05 was considered as statistically significant in two-tailed calculation.
Results
The age groups 41-50 is the more representative of the sample and females are slightly older than males. Put together the age class (31- 60) represents 72.35% of the population (Table 1).
Age groups
Gender of Patient
Total
Male
Female
Under 20
16
7
23
21-30
95
63
158
31-40
118
80
198
41-50
160
165
325
51-60
110
92
202
61-70
42
32
74
71 and older
11
11
22
Total
552
450
1002
Age(yr) Mean±SD
42.97±13.2
44.73±12.33
43.76±12.84
Table 1: Age and gender distribution.
Psychological factors are common in the population account for around 51.50%. Stress and anxiety are the most predominant for 23% and 17.4% respectively. These factors frequency increase from 21 to 50 years old then decrease after 50 years old, also these factors present a peak in the age group 41-50 (Table 2).
Symptoms
Gender
Age groups
Total
Percentages in population N=1002
Under 20
21-30
31-40
41-50
51-60
61-70
71 and older
Anxiety
Male
1
19
17
29
18
6
1
91
9.08%
Female
1
7
16
38
10
8
3
83
8.28%
Total
2
26
33
67
28
14
4
174
17.40%
Depression
Male
0
1
1
1
6
0
1
10
1.00%
Female
0
1
1
2
1
0
0
5
0.50%
Total
0
2
2
3
7
0
1
15
1.50%
Panic disorder
Male
1
2
5
8
2
0
0
18
1.80%
Female
0
4
4
10
3
0
0
21
2.10%
Total
1
6
9
18
5
0
0
39
3.90%
Stress
Male
2
29
39
30
18
7
1
126
12.58%
Female
1
20
29
36
14
4
0
104
10.38%
Total
3
49
68
66
32
11
1
230
23.00%
Others Psychol. conditions
Male
2
4
6
13
5
3
0
33
3.3%
Female
1
3
6
6
6
3
0
25
2.50%
Total
3
7
12
19
11
6
0
58
5.80%
Total by age groups
9
90
124
173
83
31
6
516
51.50%
Table 2: Psychological factors history distribution.
There is a male predominance in drugs intake except for 41-50, 51-50 and 71 and older age groups. Also age group 41-50 represents the peak of drugs intake among both male and female (Figure 1).
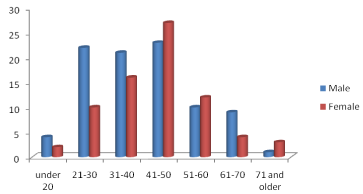
Figure 1: Drugs intake history distribution by age and gender in the
population.
Around one fifth of the population has a specific FGID. Functional constipation is more prominent in females and functional diarrhea in males; whereas FAP and IBS are slightly prominent among females and FUBD in males. The overall FGID is somewhat prominent in males (Table 3).
Type of FGID
Gender
Total
Prevalence in population (N, %)
Male
Female
Functional abdominal pain
131
117
248
24.75%
IBS
123
127
250
24.95%
Functional constipation
92
136
228
22.75%
Functional diarrhea
120
91
211
21.05%
Functional bloating
143
147
290
28.94%
Functional unspecified bowel disorder
64
55
119
11.87%
Overall FGIDs
284
274
558
55.7%
Table 3: Distribution of FAP and FBDs in the population (N=1002).
NB: We found that a number of 97 patients (9.68%) who have normal colonoscopic results but didn’t fulfill the Rome III diagnostic criteria for FGID due to symptoms onset duration mismatch, these patients are called “unclassified patients”
The overlapping of FGIDs is common. The proportion of subjects that have two coexisting FGID is 29.39%, those having three 25.09% and those having more than three 17.56% (Table 4).
Number of FGID
Number of patients (n, %)
Percentage in population (N, %)
1
156 (27.96% )
15.56%
2
164 (29.39%)
16.37%
3
140 (25.09%)
13.97%
4
57 (10.21%)
5.69%
5
31 (5.55%)
3.09%
6
10 (1.8%)
0.99%
Total
558 (100%)
55.7%
Nearly 72.04% of patients had multiple FGID while 27.96% had just one FGID
Table 4: The Overlap among different FGID, (N=1002).
The peak of FGID is observed in age group 41-50, also FGIDs increase from under 20 to 41-50 then decrease after 41-50 years respectively (Table 5).
FGIDs Gender
Age groups
Total
N,% (N=1002)
Under 20
21-30
31-40
41-50
51-60
61-70
71 and older
Male (n=552)
7
49
72
82
52
20
2
284
28.34
Female (n=450)
4
37
57
98
52
20
6
274
27.34
Total
11
86
129
180
104
40
8
558
55.7
FGIDs & Gender Chi2test P value=0.003 and FGIDs & Age Chi2test P value=0.048
Table 5: Distribution of overall FGIDs by age groups and gender.
There is a statistically significant relationship between IBS and gender, IBS and patient’s age. The frequency increases from under 20 years to 41-50, then decreases progressively (Table 6).
IBS Gender
Age groups
Total
N, % (N=1002)
Under 20
21-30
31-40
41-50
51-60
61-70
71 and older
Male
3
27
29
34
22
8
0
123
12.28
Female
1
20
30
39
28
6
3
127
12.67
Total
4
47
59
73
50
14
3
250
24.95
IBS & Gender Chi2test P value=0.031 and IBS & Age Chi2test P value=0.027
Table 6: IBS distribution by gender and age groups.
IBS-diarrhea is the more frequent subtype without sex predominance; IBS-constipation and mixed-IBS are prominent in female gender while unsubtyped IBS is in male (Table 7).
Subtype
Male
Female
Frequency (N)
IBS Constipation
49
74
123
IBS Diarrhea
73
73
146
IBS Mixed
38
55
93
Unsubtyped IBS
64
61
125
Table 7: Distribution of IBS subtyping through gender based on Bristol stools form.
Stress has the strongest relationship with IBS, then follows FUBD, FAP, Fc and Fb decreasingly but has no association with Fd.
Depression has the strongest relationship with FAP then follows FUBD and Fb but have no relationship with IBS, Fd and Fc.
Anxiety and other psychological conditions have no relationship with FGIDs while panic disorder has relationship with FUBD and finally drugs intake has relationship with FAP (Table 8).
Fd
Fc
Fb
FAP
IBS
FUBD
Stress (n=230)
44(19.1%)
67(29.1%)
80(34.8%)
72(31.3%)
75(32.6%)
39(16.9%)
P value
0.414
0.009**
0.026*
0.009**
0.002**
0.007**
Depression (n=15)
4(26.6%)
4(26.6%)
8(53.3%)
10(66.6%)
5(33.3%)
6(40%)
P value
0.591
0.716
0.036*
0.001**
0.45
0.001**
Anxiety (n=174)
40(23% )
36(20.7% )
47(27% )
42(24.1%)
48(27.6%)
27(15.5%)
P value
0.492
0.475
0.537
0.837
0.377
0.102
Panic disorder(n=39)
8(20.5% )
10(25.6% )
11(28.2%)
14(35.9%)
9(23.1% )
10(25.6%)
P value
0.932
0.661
0.918
0.1
0.783
0.007**
Others Psychological cond.(n=58)
14(24.1%)
11(19% )
16(27.6%)
14(24.1%)
10(17.2%)
5(8.6%)
P value
0.553
0.478
0.815
0.911
0.162
0.43
Drugs intake( n=164)
38(23.2%)
34(20.7% )
55(33.5%)
53(32.3%)
44(26.8%)
22(13.4%)
P value
0.468
0.5
0.156
0.014*
0.543
0.505
*=P<0.05 , **=P<0.01, P value = drawn from Chi2test
Table 8: Psychological factors and drugs intake relationship with different FGID.
This result shows us that only stress is a significant risk factor of FGIDs in our population (Table 9).
Dependent variable (FGIDs)
Significance
OR
95% CI for OR
low bound
upper bound
Factors variables
anxiety
0.643
0.924
0.660
1.292
depression
0.065
3.352
0.929
12.090
panic disorder
0.488
0.789
0.404
1.541
stress
0.049
1.360
1.002
1.847
other psych.
0.418
0.802
0.470
1.368
Drugs intake
0.391
1.162
0.825
1.636
Table 9: Relative risk of FGIDs if having psychological factors and drugs intake history.
Discussion
Prevalence
Taking into account the number of criteria required to meet the definition of each disorder, the prevalence varies greatly under method, sample size, criteria used for diagnosis, type of population, geographic location, country etc. Minor changes in definition can change all the estimates.
Prevalence of overall FGIDs (FBDs and FAP) and overlapping syndrome: The overall FGID diagnosed is estimated at 55.7% in the population what demonstrates that FGID are common in this population. Indeed half of all adults who suffer from chronic abdominal pain and stools irregularity have functional bowel disorders according to Winfried et al. [10].
Studies in Japan outpatients [11] and china adolescents [7] found comparable results respectively 57.4% and 55.24%. However our rate is higher than those of Linda [12], Walsh [13], Liu [14], Fang-Yuan [15], Moghimi-Dehkordi [16] and Kheng-Seong [9] who found up to 40%, 41.2%, 27.8%, 26.2%, 10.9% and 33% respectively; but less than that of Thompson [8] who found 61.7%. These variations can be explained by the heterogeneity in measured outcomes, study design, samples size, symptoms definitions, indications for colonoscopy and/ or inclusion criteria, which may also reflect the discrepancies in the evolution of the definitions, and the still unknown etiologies of these nonspecific symptoms. Direct comparisons of results between studies, as well as generalization and recommendations for all individuals with FGIDs are therefore difficult [17].
Our study is conducted among hospital outpatient patients; this can make a big difference with population based studies which use bigger sample size and where subjects included did not seek for a medical care. We have also focused on two categories of FGIDs: FBD (C1-C5) and FAP (D1) among the six major categories of FGID which equals to six individuals FGIDs, this reason also can explain our rate. Findings in whole population may be quite different from findings in patients population in which the individual syndromes may be stable and less prone to transitions between syndromes [18]. Patient-based studies from health institutions are inherently biased by health care seeking because almost half of subjects consult a health care provider regarding their symptoms [12].
As FGIDs varies depending on the diagnostic criteria, the geographic area of evaluated population, age, gender and environmental factors; racial and cultural differences are also important to take into consideration. Indeed, studies revealed a greater prevalence of FGIDs in western countries than others and concomitantly FGIDs are more common in industrialized city than non industrialized city [19].
In a study by Ghoshal et al., while comparing the percentage of subjects fulfilling different diagnostic criteria for the same FGID in the same sample it was found rates for Manning: 91.122%, Rome I: 67.9% , Rome II: 40.1% ,Rome III: 52.5% and Asian criteria: 74.5% among 1618 patients; what proves the variation of prevalence through diagnostic criteria. The Rome III criteria were less restrictive and showed good agreement with the Rome II criteria. Considering all these above mentioned factors, it becomes very difficult or virtually impossible to compare prevalence rates from different time periods or geographic regions.
Overlapping among FGIDs is very common in this study and was estimated at 72.04% of total FGIDs with 29.39% having two coexisting FGID and 25.09% having three coexisting FGID. Comparatively Xiong [20] found 50.3% of patients with overlapping disorders with 37.4% having two coexisting FGID, 8.9% having three coexisting FGID, while Fang-Yuan [15] found 25.7% of overlapping between functional dyspepsia and other FGIDs and Nakajima [11] 42.6% of overlapping FGID with 29.6% having two coexisting FGID and 11.1% having 3 coexisting FGID. This phenomenon of overlapping implies that all the FGIDs may share a mutual underlying pathophysiology as they happen in the same patient and improvement of other symptoms is observed when treating one FGID [21]. Also, the flexibility of Rome III criteria allows this overlapping such that borders are blurred between disorders. Studies are increasingly supportive of the possibility that these disorders are multifactorials [22]. A commonly held perception is that FGIDs are chronic stable conditions, although symptoms may wax and wane [18]. Many episodes of symptom disappearance were due to subjects changing symptoms rather than total symptom resolution, this transition between FGIDs suggests a common etiopathogenesis. Among people with symptoms at baseline, approximately 20% had the same symptom, 40% had no symptoms, and 40% had different symptoms at follow-up [18].
IBS: IBS, the best known and most studied among FGIDs accounts for 20-50% of all gastroenterology consultations [23] and 20-50 % of referrals to gastroenterology clinic [24]. Epidemiological studies worldwide reported a prevalence of 6-25% of IBS [3]. However, disparities exist between countries and regions of the world, better still between sex and age.
Prevalence of IBS was 24.95% in our study. Similar studies found 25% in Canada [25], 23.4% in China [26] and 27 % [27] in Iran. Some authors found lower rate than ours: 4.4% [15], 6.90% [7], 11.1% [9], 17.2% [12], 10-20% [23], 18.8% [28] and others higher 32.5% [29], 40.2% [20], 47.1% [30], 70.3% [13].
Differences of prevalence are remarkable between several studies, these are unlikely due to true inter-country variations but rather than to different sociocultural perceptions and reporting of symptoms by subjects, or to different interpretations of symptoms by interviewers , or to the lack of correspondence in any single language between the native lay verbal definition of symptoms and the translated terminology [1]. Most studies indicate that the prevalence of IBS is higher in women than in men and in adults and elderly than in young subjects [2,29,31,32] also, the prevalence of IBS was decreasing with ageing [1].
In this study we found that IBS is highest in the age group 41-50 and decrease progressively after 50 years. This is comparable to a study by Adibi et al. who found that across Asia IBS prevalence is higher in the younger age groups, applying Rome II and it is significantly more prevalent in those below 50 years of age than those of 50 years and older.
While sub typing IBS based on the stool form we found IBSconstipation 25.25%, IBS-diarrhea 30%, mixed-IBS 19.1% and otherwise a 1:1 ratio. Comparatively in 2011 another study in outpatients in Wuhan found 10.7% of IBS , a 1:1 ratio, C-IBS 30.8% and D-IBS 45.2% by using Rome II criteria [26]. As in our study IBSdiarrhea is particularly most frequent in Asia and conversely IBSconstipation in European countries. One possible explanation may be the low-fiber diet in western cooking. The differences in results in IBS sub typing may be due to the diagnostic criteria, as a recent study from China that compared the Rome II and III criteria found the latter to be better [33]. Also there is poor agreement between sub typing of IBS patients based on Rome II versus Rome III criteria [34, 35].
Functional Abdominal Pain (FAP): The prevalence of Functional Abdominal Pain was 24.75% in this study. Linda [12] in 2006 found a rate greater than ours 33.3%, while others authors [8,13,18,20,36] lesser than ours: 20.2% (USA), 13-17% (USA), 7.9% (Ireland), 2.7% (Canada), 2.3% (China) respectively. This difference could be explained by the method used, sample size, nature of sample (patients or general population) and criteria used to define FAP. Basically it is easier to find higher prevalence in patient-based study than in population-based study. Better, it is established that sociocultural factors influence the pain behavior as do psychological factors, in our study psychological stress was found to have a strong link with FAP. Also, the combination of genetic factors, vulnerability factors, and adult stress may determine in part the effectiveness of endogenous pain modulation systems and thereby influence the development of FAPS [37]. Diagnosing a patient who presents with abdominal pain can be challenging since it can be difficult to properly evaluate these patients without overusing diagnostic tests and consultation [38]. To the same extent, children with FAP have a high utilization of health care system as, they, along with their parents; seek answers for the unexplained abdominal pain. Pain interferes with normal attendance and performance at school, peer relationships and participation in family activity. Fortunately, FAP is uncommon under 4-6years [36]. Increasing evidence from limited studies support that the morbidity associated with FGID is psychosocial [36].
In our study FAP is more common in male than in female although it is not statistically significant. However several studies [1,5,6,28,37,39,40,] indicate that FAP is more frequent in women and associated with significant work absenteeism and physician visits. This gender distribution of FAP is still not clear.
Functional diarrhea (Fd): We found a prevalence of 21.05% for functional diarrhea in this study. Other authors [7- 9,12,13,18,20,30,40] found 0.70%, 1.1%, 1.5%, 1.5%, 3.6%, 3.7%, 5.7%, 8.5%, 25.1% respectively. It is evident that prevalence widely differs through studies; this may be due to factors included and/or criteria used to diagnose this FGID.
For unknown or unclear reason diarrhea seems to be more frequent and troublesome symptom in men than women, also majority of FGID studies supports this fact, while other FGID symptoms were predominant in female [7,11,30,41].
Fd have a lower rate compare to other FGIDs, this is most likely due to IBS as that diarrhea is also part of criteria for its diagnosis. A study [9] had revealed that male gender and age > 60 years are predictive of diarrhea. Possible explanation may be that physiologically men’s colon transit is more rapid than women’s, although psychological distress, drugs, food intolerance can induce diarrhea. Understanding of Fd is limited because few studies had put interests in it compared to FD or IBS.
Functional constipation (Fc): Functional constipation prevalence was 22.75% in our study. By using Rome II or III criteria and diverse methods, increasingly some authors found a lower rate 2.1% [12], 4.1% [18], 4.4% [15], 8.1% [9], 8.1% [9], 11% [30], 12.6% [20], 14.9% [8], 3-16.7% [1], 16.95% [7], 22.5% [13] while others a higher rate 25.92% [42], 28% [27] compared to ours.
The prevalence of constipation varies with the different definitions used and in the different populations investigated. In our study we found an increase in prevalence with age and it is more frequent in adult female than in adult male as did Corazziari’s [1]. For Thompson et al. [31] constipation occurs in up to 20% of populations, depending on demographic factors, sampling and the definition used. Also literature stated that female sex, older age, inactivity, low caloric intake, taking a large number of medication, low-fiber diet, low income and low education levels could be risk factors of constipation. The incidence of constipation is three times higher in women, and women are twice as likely as men to schedule physician visits for constipation [40,17]. Studies have shown that bowel transit time in women tends to be slower than in men, and many women experience constipation during their menstrual period [43]. Environmental factors like living in rural areas and in colder temperatures, geographic localization and cultural eating habits can increase susceptibility to constipation. Fc in older adults may result from autonomic neuropathies, such as diabetes mellitus and Parkinson disease, or from use of medications, such as antacids especially with calcium, opioids, iron supplements and anticholinergics or from conditions such as depression, hypothyroidism, cerebrovascular disease and IBS [43].
Functional Unspecified Bowel Disorder (FUBD): The prevalence of functional unspecified bowel disorder was 11.87 % in this study. Comparatively the rate was lower in some studies 3.8% [30], 8.9% [15] and higher in others 13.9% [20], 18% [27], 26.58% [7], 38.7% [11] than ours. The difference lies in the choice of criteria (Rome II or III), the sample size, location of the study as well as nature of the population (patients, general population, city dweller, peasant, high education level, student). Most of the time this disorder is confounded with IBS, as is the case of Nakajima study [11] where FUBD have been reported to be the most prevalent (38.7%) bowel disorders. However, this FGID is uncommon as other FGIDs should be excluded before you are declared FUBD.
Functional bloating (Fb): Bloating is one of the most common and bothersome symptoms complained by a large proportion of patients [36]. In this study we found a Fb prevalence of 28.94%. The prevalence varies among studies 2.6% [20], 4.12% [7], 6.1% [9,40], 7% [44], 8.2% [13], 9.1% [30], 13.1% [8], 25% [27] thus our rate was highest. Prevalence’s rates vary widely, depending on diagnostic criteria and other factors. Epidemiologically, one in six to one in five healthy individuals reported bloating in population-based studies both in Western and East countries [45]. Thompson et al. [31] found 15% in a population based studies and bloating was more prevalent in women. Indeed there are great difficulties in terms of diagnosis due to lack of appropriate parameters that grade and assess bloating. It is still unclear to what extent the individual patient complaint of subjective bloating correlates with the objective evidence of abdominal distension.
Tuteja [44] stresses that bloating is a common symptom in otherwise healthy adults, and is often associated with but not predictive of functional bowel disorders and that smoking and highdose aspirin are associated with bloating.
FGIDs relation with age and gender
Our study has revealed a significant relationship between FGIDs and patient’s age and sex in general, and particularly in IBS (Table 5, 6). Indeed, there is difference in age and gender distribution among patient with FGID among studies. In ours, the age group (41- 50) years, is the largest but for Nakajima [11] (70-79) years was the largest. Mean of age also is different 57.8 years for [11] while for us it is 43.72 years. Other studies using Rome III criteria found a mean of age not far from ours, Tang [30], 45.55±10.68 and Liu [14] 44.36±0.35. Despite difference in methods between studies, the age related high frequency of disorders in [30-60years] in our case, is comparable to several studies’ [11,14,30,] as both normal physiological changes and pathological conditions are related to age. Consequently, the occurrence of symptoms is likely to vary in different age groups. In our study we found that FGIDs decrease with ageing, as did Fang-Yuan et al. [15]. Age may significantly be related to the prevalence of FGIDs, for example Chang [41] found that IBS, FAP, Fd decreased with age while Fc increased with age, and discordance for Fb. It is thought that the high prevalence among young adult is due to psychological factors as they are influenced by studying, job-seeking, or economic status [15]. Functional bowel symptoms nonetheless are common in the elderly, in whom they are more likely to be misdiagnosed or attributed to organic findings of uncertain significance [46].
Likewise majority of studies found that FGID is more prevalent in women, while other few studies found equal or male prevalence in FGID distribution. Corazziari [1] reported a 2:1 female: male ratio for chronic abdominal pain and constipation, a 1:1.5 male predominance for functional bloating. Others authors [7,10,15,41] found a greater prevalence of FGIDs in female except for functional diarrhea. We also found functional diarrhea to be more prominent in male in our study. According to Chang et al. [41] there is female sex hormone effect on patients with IBS in visceral pain perception and on psychological measures (Female > Male).
There is discordance in gender prevalence of IBS in Asia: while some countries have male predominant prevalence (Mumbai and Pan-India, Korea), others female predominant prevalence (Japan) and equal prevalence Pakistan and China [47]. For Husain et al. [48] the equal sex ratio of IBS in urban Pakistan could result from a close association between marked distress and IBS in men similar to that found in women in western studies. A FGID study in Taiwan general population [28] revealed that subjects affected were younger, had less vegetables and fruits intake, higher BSRS (brief-symptom rating scale) score and were of greater female predominance. Grodzinsky et al. [19], found that the gender difference might be randomly due to an unknown factor or to the fact that more women suffered from IBS and seek healthcare more often when their children have the same GI complaints.
Another study by Hammer [15] revealed that constipation and bloating were more frequent in females independently whether they have IBS or organic disease; all the diagnostic criteria for IBS had higher predictive value in females compared to males. The possible explanation for the apparent sex specificity in IBS includes the following: differences in symptom perception, GI function, or the socially learned response to symptoms by sex, difference in symptomatic response to treatment between sexes, women having slower gut transit times which explain why they report less frequent stools and a higher prevalence of constipation [29,41,49].
FGIDs relation with drugs intake
In this study 16.4% of the population reported a drug intake, out of them 58. 54 % have a FGID. However we found no correlation between drugs intake and FGIDs occurrence (P=0.42) unless in FAP (P=0.014). Through literature [23,50,51] drugs-induced GI symptoms are recognized and drugs like laxatives, NSAIDs, steroids, calcium antagonists, antiacids, antidepressant, iron pills, narcotics could cause FGIDs. Indeed, long-term narcotic use can cause the Narcotic Bowel Syndrome (NBS), a chronic or periodic abdominal pain that gets worse when the effect of the narcotic drug wears down. For Bhat [52], the likelihood of symptoms being functional increased even further if adverse reactions to both drugs and foods were reported. Patients with weight gain were more likely to report food allergy, and those with both features were very likely to have a functional disorder (OR: 4.58, 95% CI: 3.08–6.86) [52]. Patients with gastrointestinal symptoms who report drug or food allergies or worsening of symptoms with various foods are more likely to have functional than organic illness [52].
FGIDs and psychological factors
Studies have corroborated this association for a while. In our study we found prevalence of stress 25.27%, anxiety 17.03 %, depression 2.15%, panic disorder 3.77 % and others psychological conditions 5.2% however their degree of relation with FGIDs is variable. For example stress is significantly related to IBS (P=0.002), FUBD (P=0.007), FAP (P=0.009), Fc (P=0.009), Fb (P=0.026) and depression to FAP (P=0.001), FUBD (P=0.001), Fb (P=0.036). Routinely psychological factors had higher close relationships with FBDs [7] and FAP [38] than others FGIDs. Psychological factors have been reported in Chinese studies as in western studies to play a role in the pathogenesis of IBS [26]. A study by Monnikes et al. [53] found that stress induces differential motor effects on the upper and lower GI tract in healthy human subjects, better the role of stress and stressful events is well recognized in patients with FGID [54,55]. More precisely Mussel [39] found that the prevalence of severe levels of depression was nearly fivefold in patients with GI symptoms compared to patients without GI symptoms (19.1% vs. 3.9%; Pb.001), and the prevalence of severe levels of anxiety was nearly fourfold in patients with GI symptoms compared to patients without GI symptoms (19.4% vs. 5.6%; Pb.001). Psychological stress is widely believed to play a major role in IBS by precipitating exacerbation of symptoms. Body of evidence from experimental studies suggest that the central nervous system CNS response to stress modulates the autonomic nervous system outflow, activates the hypothalamicpituitary- adrenal axis and alters pain modulator mechanisms, these effects can be associated with changes in GI motility and visceral sensitivity [53]. Thus illness behaviors, life stress, psychosocial factors understanding were important in treatment as they are predictors of favorable outcome [49]. Indeed women with FGID appear to respond well to psychological treatment while men have shown less response [41]. Additionally another study by V. Lee et al. [56] confirmed that psychological factors are significantly associated with health-related quality of life in patients with IBS in primary care. In our study, stress and depression have a higher relationship with IBS compared to the remaining FGIDs. Drossman [57] found that in IBS patients, the most co-morbid psychiatric disorders were anxiety, mood disorder, and somatoform disorder. Anxiety and depression were found to be related to FAP [37] and IBS [58]. In opposition John [28] found no difference in rates of psychiatric illness in subjects according to the presence of functional GI symptoms.
There is evidence that colon is more sensitive to stress than other parts of GI tract, whether this explain the effects of stress in IBS and why IBS is the most common functional bowel disorder is unclear. Emotional distress may either stimulate or inhibit motility, contributing to diarrhea or constipation in the 30% of the US population with IBS [39]. Anxiety, major depression, social phobia, panic disorder, somatization etc. have been identified in more than 50% of patients with IBS [38]. IBS appears to be part of a continuum of GI and CNS reactions to external and internal stimuli and many people have functional GI symptoms in response to emotional stress [59].
The strength of our study is that the questionnaires were filled in front of a doctor or a trained assistant what resolve eventual misunderstanding immediately, contrary to a retrospective study or survey (mailed questionnaire). In addition all the enrolled subjects have undergone colonoscopy what improve our FGIDs diagnostic accuracy. More we have obtained “true FGIDs” prevalence unlike symptom-based diagnostic method which relies on absence of alarm symptoms and meeting of Rome III criteria.
The limit of our study is its patient-based footprint, while a population-based study will inform us better on a large sample distribution and in non healthcare seeking subjects such that the majority of FGIDs patients will not consult a practitioner for their symptoms. Also it is a transversal study so we don’t have any idea on the natural history and evolution of symptoms over the time compare to a follow up study.
Conclusion
Our study has revealed that 55.7% of symptomatic GI patients have a FGID; around 10% of patients are “unclassified patients”; a high overlapping among different FGIDs (72.04%). FGIDs are common and data have showed that FGIDs are strongly related to gender, we also found that these disorders are more frequent among under-50 years old and decrease after 50 years. There is relationship between FGIDs and psychological factors like stress and depression, a just as there is correlation in some drugs intake and the occurrence of FAP. Stress is found to be a risk factor for FGIDs. There is need for further studies to evaluate less investigated FGIDs such Fd, Fb, FUBD for better knowledge.
References
- Enrico Corazziari. Definition and epidemiology of functional gastrointestinal disorders. Best Practice & Research Clinical Gastroenterology. 2004; 18: 613–631.
- Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010; 25: 252-258.
- Gschossmann JM, Haag S, Holtmann G. Epidemiological trends of functional gastrointestinal disorders. Dig Dis. 2001; 19: 189-194.
- Douglas A, Drossman, Melissa swantkowski. History of functional disorders. UNC, Center for Functional GI and motility disorders.
- Lee OY, Schmulson M, Mayer EA. Common functional gastrointestinal disorders: nonulcer dyspepsia and irritable bowel syndrome. Clin Cornerstone. 1999; 1: 57-71.
- Guarner, Lazaro, Gascon, Royo, Eximan, Herrero. Map of Digestive Diseases and Disorders (MDD). World Gastroenterology Organization. 2008.
- Chu L, Zhou H, Lü B, Li M, Chen MY. An epidemiological study of functional bowel disorders in Zhejiang college students and its relationship with psychological factors. Zhonghua Nei Ke Za Zhi. 2012; 51: 429-432.
- Thompson WG, Irvine EJ, Pare P, Ferrazzi S, Rance L. Functional gastrointestinal disorders in Canada: first population-based survey using Rome II criteria with suggestions for improving the questionnaire. Dig Dis Sci. 2002; 47: 225-235.
- Ng KS, Nassar N, Hamd K, Nagarajah A, Gladman MA. Prevalence of functional bowel disorders and faecal incontinence: an Australian primary care survey. Colorectal Dis. 2014.
- Häuser W, Layer P, Henningsen P, Kruis W. Functional bowel disorders in adults. Dtsch Arztebl Int. 2012; 109: 83-94.
- Nakajima S, Takahashi K, Sato J, Fukuda M, Yamamoto K, Inoue T, et al. Spectra of Functional Gastrointestinal Disorders Diagnosed by Rome Iii Integrative Questionnaire in a Japanese Outpatient Office and the Impact of Overlapping. J Gastroenterol Hepatol. 2010; 25: S138-S143.
- Olafsdottir LB, Gudjonsson H, Jonsdottir HH, Bjornsson E, Thjodleifsson B. Natural history of functional gastrointestinal disorders: comparison of two longitudinal population-based studies. Digestive and Liver Disease. 2012; 44: 211–217.
- Walsh K, McWilliams SR, Maher MM, Quigley EM. The spectrum of functional gastrointestinal disorders in a tertiary referral clinic in Ireland. Ir J Med Sci. 2012; 181: 81-86.
- Liu Jinsong, Huang Hong. Comparison of clinical characteristics between Functional bowel Disorders and Organic Bowels Diseases. Chin J Gastroenterol. 2009; 14: 738-741.
- Chang FY, Chen PH, Wu TC, Pan WH, Chang HY, Wu SJ, et al. Prevalence of functional gastrointestinal disorders in Taiwan: questionnaire-based survey for adults based on the Rome III criteria. Asia Pac J Clin Nutr. 2012; 21: 594-600.
- Moghimi-Dehkordi B, Vahedi M, Pourhoseingholi MA, Khoshkrood Mansoori B, Safaee A, Habibi M, et al. Economic burden attributable to Functional Bowels Disoders in Iran: A cross-sectional population-based study. Journal of Digestive Diseases. 2011; 12; 384–392.
- Schusselé Filliettaz S, Gonvers JJ, Peytremann-Bridevaux I, Arditi C, Delvaux M, Numans ME, et al. Appropriateness of colonoscopy in Europe (EPAGE II). Functional bowel disorders: pain, constipation and bloating. Endoscopy. 2009; 41: 234-239.
- Halder SL, Locke GR, Schleck CD, Zinsmeister AR, Melton LJ, Talley NJ. Natural History of Functinal Gastrointestinal disorders: A 12 years longitudinal population-Based study. Gastroenterology. 2007; 133: 799-807.
- Ewa Grodzinsky, Claes Hallert, Tomas Faresjo, Elisabet Bergfors, Ashild Olsen Faresjo. Could gastrointestinal disorder differ in two close but divergent social environments?” International Journal of health Geographics. 2012, 11: 5.
- Xiong LS, Shi Q, Gong XR, Cui Y, Chen MH. The spectra, symptom profiles and overlap of Rome III functional gastrointestinal disorders in a tertiary center in South China. J Dig Dis. 2014; 15: 538-544.
- Park H. Functional gastrointestinal disorders and overlap syndrome in Korea. J Gastroenterol Hepatol. 2011; 26 Suppl 3: 12-14.
- SE Kim, L Chang. Overlap between functional GI disorders and other functional syndromes: what are the underlying mechanisms?. Neurogastroenterol Motil. 2012; 24: 895–913.
- Grassi M, Petraccia L, Mennuni G, Fontana M, Scarno A, Sabetta S, et al. Changes, functional disorders, and diseases in the gastrointestinal tract of elderly. Nutr Hosp. 2011; 26: 659-668.
- Delvaux M. Functional bowel disorders and irritable bowel syndrome in Europe. Aliment Pharmacol Ther. 2003; 18: 75-79.
- Li FX, Patten SB, Hilsden RJ, Sutherland LR. Irritable bowel syndrome and health-related quality of life: a population-based study in Calgary, Alberta. Can J Gastroenterol. 2003; 17: 259-263.
- Liu J, Hou X. A review of the irritable bowel syndrome investigation on epidemiology, pathogenesis and pathophysiology in China. J Gastroenterol Hepatol. 2011; 26 Suppl 3: 88-93.
- Roshande D, Rezailashkajani M, Shafaee S, Zali MR. Symptom patterns and relative distribution of functional bowel disorders in 1,023 gastroenterology patients in Iran. Int J Colorectal Dis. 2006; 21: 814-825.
- Wyeth JW. Functional gastrointestinal disorders in New Zealand. J Gastroenterol Hepatol. 2011; 26: 15-18.
- Hammer J, Talley NJ. Value of different diagnostic criteria for the irritable bowel syndrome among men and women. J Clin Gastroenterol. 2008; 42: 160-166.
- Tang YR, Wang P, Yin R, Ge JX, Wang GP, Lin L. Five-year follow-up of 263 cases of functional bowel disorder. World J Gastroenterol. 2013; 19: 1466-1471.
- Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Müller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999; 45: II43-II47.
- Park DW, Lee OY, Shim SG, Jun DW, Lee KN, Kim HY, et al. The Differences in Prevalence and Sociodemographic Characteristics of Irritable Bowel Syndrome According to Rome II and Rome III. J Neurogastroenterol Motil. 2010; 16: 186-193.
- Yao X, Yang YS, Cui LH, Zhao KB, Zhang ZH, Peng LH, et al. Subtypes of irritable bowel syndrome on Rome III criteria: a multicenter study. J Gastroenterol Hepatol. 2012; 27: 760-765.
- Ghoshal UC, Abraham P, Bhatia SJ, Misra SP, Choudhuri G, Biswas KD, et al. Comparison of Manning, Rome I, II, and III, and Asian diagnostic criteria: Report of the Multicentric Indian Irritable Bowel Syndrome (MIIBS) study. Indian J Gastroenterol. 2013; 32: 369–375.
- Ersryd A, Posserud I, Abrahamsson H, Simrén M. Subtyping the irritable bowel syndrome by predominant bowel habit: Rome II versus Rome III. Aliment Pharmacol Ther. 2007; 26: 953-961.
- Ammoury RF, Pfefferkorn Mdel R, Croffie JM. Functional gastrointestinal disorders: past and present. World J Pediatr. 2009; 5: 103-112.
- Clouse RE, Mayer EA, Aziz Q, Drossman DA, Dumitrascu DL, Mönnikes H, et al. Functional abdominal pain syndrome. Gastroenterology. 2006; 130: 1492-1497.
- Holten KB, Wetherington A, Bankston L. Diagnosing the patient with abdominal pain and altered bowel habits: is it irritable bowel syndrome? Am Fam Physician. 2003; 67: 2157-2162.
- Mussell M, Kroenke K, Spitzer RL, Williams JB, Herzog W, Lowe B. Gastrointestinal symptoms in primary care: prevalence and association with depression and anxiety. J Psychosom Res. 2008; 64: 605-612.
- Wadsworth CA, Olivia Li J, Thillainayagam AV. Symptoms and signs of lower gastrointestinal disease. Elsevier, Medecine. 2011; 39: 72-78.
- Chang L, Toner BB, Fukudo S, Guthrie E, Locke GR, Norton NJ, et al. Gender, age, society, culture, and the patient's perspective in the functional gastrointestinal disorders. Gastroenterology. 2006; 130: 1435-1446.
- Zhou HQ, Li DG, Song YY, Zhong CH, Hu Y, Xu XX, et al. An epidemiologic study of functional bowel disorders in adolescents in China. Zhonghua Yi Xue Za Zhi. 2007; 87: 657-660.
- Jamshed N, Lee ZE, Olden KW. Diagnostic approach to chronic constipation in adults. Am Fam Physician. 2011; 84: 299-306.
- Tuteja AK, Talley NJ, Joos SK, Tolman KG, Hickam DH. Abdominal bloating in employed adults: prevalence, risk factors, and association with other bowel disorders. Am J Gastroenterol. 2008; 103: 1241-1248.
- Iovino P, Bucci C, Tremolaterra F, Santonicola A, Chiarioni G. Bloating and functional gastro-intestinal disorders: where are we and where are we going? World J Gastroenterol. 2014; 20: 14407-14419.
- Jenny Gunnarsson and Magnus Simrén ”Efficient diagnosis of suspected functional bowel disorders.” Nature Clinical Practice Gastroenterology & Hepatology. 2008; 5: 498-507.
- Gwee KA, Lu CL, Ghoshal UC. Epidemiology of irritable bowel syndrome in Asia: something old, something new, something borrowed. J Gastroenterol Hepatol. 2009; 24: 1601-1607.
- Husain N, Chaudhry IB, Jafri F, Niaz SK, Tomenson B, Creed F. A population-based study of irritable bowel syndrome in a non-Western population. Neurogastroenterol Motil. 2008; 20: 1022-1029.
- Jones MP, Crowell MD, Olden KW, Creed F. Functional gastrointestinal disorders: an update for the psychiatrist. Psychosomatics. 2007; 48: 93-102.
- David S. Greenbaum, “Lower GI tract and its common functional disorders, IBS, Chronic Functional Abdominal Pain, Bloating and Gas, Constipation, Diarrhea.
- Caporaso N, Morisco F, Penagini R. Functional intestinal disorders: how to improve diagnosis and treatment in general practice. Minerva Gastroenterol Dietol. 2010; 56: 101-120.
- Bhat K, Harper A, Gorard DA. Perceived food and drug allergies in functional and organic gastrointestinal disorders. Aliment Pharmacol Ther. 2002; 16: 969-973.
- Mönnikes H, Tebbe JJ, Hildebrandt M, Arck P, Osmanoglou E, Rose M, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001; 19: 201-211.
- Nicholl BI, Halder SL, Macfarlane GJ, Thompson DG, O'Brien S, Musleh M, et al. Psychosocial risk markers for new onset irritable bowel syndrome--results of a large prospective population-based study. Pain. 2008; 137: 147-155.
- Blanchard EB, Lackner JM, Jaccard J, Rowell D, Carosella AM, Powell C, et al. The role of stress in symptom exacerbation among IBS patients. J Psychosom Res. 2008; 64: 119-128.
- Lee V, Guthrie E, Robinson A, Kennedy A, Tomenson B, Rogers A, et al. Functional bowel disorders in primary care: factors associated with health-related quality of life and doctor consultation. J Psychosom Res. 2008; 64: 129-138.
- Drossman DA, Creed FH, Olden KW, Svedlund J, Toner BB, Whitehead WE. Psychosocial aspects of the functional gastrointestinal disorders. Gut. 1999; 45: II25- II30.
- Hausteiner-Wiehle C, Henningsen P. Irritable bowel syndrome: relations with functional, mental, and somatoform disorders. World J Gastroenterol. 2014; 20: 6024-6030.
- Longstreth GF. Definition and classification of irritable bowel syndrome: current consensus and controversies. Gastroenterol Clin North Am. 2005; 34: 173-187.