
Research Article
Austin J Gastroenterol. 2015; 2(5): 1052.
A Risk Matrix Model for the Prediction of Intestinal Tuberculosis and Differentiation from Crohn’s Disease
Larsson G1,2*, Shenoy KT³, Ramasubramanian R4, Thayumanavan L5, Balakumaran LK³, Cvancarova M6, Bjune GA7 and Moum BA8
¹Department of Medicine, Lovisenberg Diaconal Hospital, Norway
²Faculty of Medicine, University of Oslo, Norway
³Population Health and Research Institute, Medical College, India
4Thoothukudi Government Medical College, India
5Madurai Medical College, India
6Oslo University Hospital, Department of Oncology, Norway
7Institute of Health and Society, University of Oslo, Norway
8Department of Gastroenterology and Hepatology, Oslo University Hospital Ullevål, Norway
*Corresponding author: Larsson G, Unger-Vetlesen Institute, Department of Medicine, Lovisenberg Diaconal Hospital, NO-0440 Oslo, Norway
Received: July 14, 2015; Accepted: September 07, 2015; Published: September 09, 2015
Abstract
Background: Intestinal Tuberculosis (ITB) can be difficult to distinguish from Crohn’s Disease (CD), especially in resource-limited areas. By combining independent risk factors measured at diagnosis, we aimed to construct a visual risk matrix model that could predict ITB.
Methods: Treatment naïve patients with ITB (n=38) and CD (n=37) were prospectively recruited from routine clinical practice in four Indian medical centres between October 2009 and July 2012.Records from case histories, clinical examination, endoscopy and histopathology of biopsies were collected prior to sampling for faecal- and serum calprotectin and C-reactive protein. Patients with malignancy, human immunodeficiency virus infection or age below 18 years were excluded from the study.
Risk factors associated with ITB and CD diagnoses were identified from univariate analysis and entered into multiple models. The probabilities of ITB diagnosis were calculated for selected levels of risk factors and the results were arranged in a prediction matrix.
Results: Four variables were significantly associated with ITB or CD diagnosis and were combined in the final matrix. Predictors of ITB were weight loss, mucosal nodularity and faecal calprotectin = 200μg/g; predictors of CD were multi-segment involvement and faecal calprotectin < 200μg/g. The probability of ITB at diagnosis ranged from 19 to 91% and for CD from 9 to 81%, depending of the level of the risk factors.
Conclusion: A visual matrix model in which faecal calprotectin is combined with clinical and endoscopic risk factors could become a rapid, easy and pointof- care tool to differentiate between ITB and CD in clinics with limited resources.
Keywords: Intestinal tuberculosis; Crohn’s disease; Diagnosis; Risk factors; Calprotectin
Abbreviations
ATT: Anti-Tuberculous Chemotherapy; CD: Crohn’s Disease; CI: Confidence Interval; CRP: Creactive Protein; FC: Faecal Calprotectin; IQR: Inter-Quartile Range; ITB: Intestinal Tuberculosis; M.tb: Mycobacterium tuberculosis; POC: Point-Of-Care; rs: Spearman rank correlation coefficient; SC: Serum Calprotectin; SCORE: Systematic Coronary Risk Evaluation Chart; TB: Tuberculosis
Introduction
The presentation and pathological findings in Intestinal Tuberculosis (ITB) may vary, be nonspecific and can easily be confounded with other gastrointestinal diseases [1,2]. In Tuberculosis (TB) endemic areas, Crohn’s disease (CD) is recurrently mistaken for ITB because of similar clinical, radiological, endoscopic and histopathological appearance and because of limited information on CD epidemiology [2-4]. Conversely, in Western countries where CD is more frequently seen, the lack of awareness of ITB and the difficulty of confirming tuberculosis (TB) by bacteriological methods can cause ITB to be mistaken for CD [5-6]. Consequently, prescribing immunosuppressants with the intention to treat CD in a patient undiagnosed with TB could be catastrophic. Endoscopic features favouring ITB diagnosis are mucosal nodularity, transverse ulcers and patolous ileocoecal valve, whereas longitudinal ulcers and multisegment involvement are typical of CD. Although granuloma with caseous necrosis is pathognomonic of ITB, the majority of patients do not present with this feature on histopathology [1-9].
“Gold standard” ITB diagnostics include expensive modalities, require highly qualified staff and hence, are not readily available in economically deprived TB endemic areas. Thus, as opposed to practice in developed countries where laboratory diagnosis of ITB can usually be achieved, clinicians in financially challenged countries are often left with empiric Antituberculous Chemotherapy (ATT) as the only available diagnostic tool [3-12]. Hence, there is a demand for new, sensitive, rapid, easy and affordable Point-Of-Care (POC) diagnostics.
Faecal Calprotectin (FC) is used as a biomarker in patients with CD to monitor relapse of disease and treatment response [13]. During the last decade, POC devices for rapid FC measurements have been developed [14]. These are easy to perform, less costly and less personnel dependent than conventional enzyme-linked immuno-sorbent assays. C-Reactive Protein (CRP) may be used to investigate systemic inflammation in CD, in which increasing levels indicate a more severe disease [15]. Serum Calprotectin (SC) may be used to monitor CD patients on anti-TNFa therapy, in whom falling levels have been associated with a positive treatment response [16]. Recently, we found high levels of FC, SC and CRP in patients with ITB [17]. Because immunological mechanisms and cytokine release differ slightly between CD and ITB [10], calprotectin levels could vary between the two diseases.
The Systematic Coronary Risk Evaluation Chart (SCORE) has become one of the most widely used risk models in clinical medicine [18]. The SCORE predicts the 10-year probability of cardiovascular mortality by combining well-known risk factors at diagnosis, which are arranged into a simple visual colour matrix wherein each box corresponds to a specific risk profile. Similar visual risk matrix models have later been developed to predict the risk of advanced disease in patients with CD [19]. Recently, by prospectively including newly diagnosed patients with ITB or CD from routine clinical practice in Southern India, we established demographic, clinical and endoscopic risk factors of the two diseases [2]. By using the SCORE system as an example, and by combining the above independent risk factors with calprotectin and CRP measurements, we aimed to construct a visual risk matrix model, which could predict the diagnosis of ITB and differentiate it from CD.
Materials and Methods
Newly diagnosed and treatment naïve ITB and CD patients were prospectively recruited by senior gastroenterologists at four South Indian medical centres in a consecutive manner from October 2009 to July 2012 (Appendix) [2]. Diagnostic criteria for ITB and CD were used according to internationally published guidelines [1,20,21]. Demographic, clinical, endoscopic and histologic features were recorded by site investigators in standardized electronic questionnaires and collected in a database. Patients were scheduled for follow-up clinical visits after two and six months of treatment. Clinical remission after ATT was regarded confirmatory for ITB diagnosis. Exclusion criteria were malignancy, age below 18 years and human immunodeficiency virus infection. Samples for biochemistry were obtained prior to initiation of treatment and faeces spot samples were collected prior to or minimum three days after endoscopy. CRP was analysed in blood serum by use of CRP turbilatex assay (Spinreact, Girona, Spain) and automated turbidometry (Beckman Coulter AU480, Cal, USA) at a local ISO certified laboratory. Faecal aliquots and separated blood serum vials were stored at -20°C until analysis. FC and SC were analysed with enzyme-linked immuno-sorbent assays using EK-CAL and MRP 8/14 kits respectively, according to the manufacturer’s recommendations (Bühlmann Laboratories AG, Basel, Switzerland).
Statistics
All variables included in the analyses were recorded at diagnosis.
Due to the skewed distribution of data and limited sample size, the
continuous variables were described with medians and ranges and
crude differences between groups were assessed with Mann-Whitney
Wilcoxon tests. The categorical variables were listed as counts and
percentages and differences between groups were evaluated with Chisquare
or Fischer’s exact tests (when appropriate). The strength of
association between continuous variables was assessed by calculating
the Spearman rank correlation coefficient (r
As our aim was to quantify probabilities of ITB or CD based on observed or measured variables, we constructed a prediction matrix. First, the following risk factors were established from the results of our previous study [2]: weight loss (from onset of symptoms, qualitative); right inferior abdominal pain (on physical examination at diagnosis); multi-segment involvement (endoscopically apparent lesions in =3 of 6 pre-defined anatomic sub-divisions); and mucosal nodularity (round elevated nodules 2-6 mm in diameter detected upon endoscopy) (Table 1). All four variables were regarded as dichotomous. Then, univariate logistic regression models were fitted and variables which differed significantly between the groups (p < 0.05) were included into further analyses. Risk factors that were highly associated with each other were excluded to avoid multicollinearity. FC, SC and CRP were measured as continuous variables, followed by categorization into dichotomous variables. Several cut-off levels for FC were tested, based on both statistical properties and clinical recommendations [17-24]. Cut-off levels were evaluated separately as well as combined with the previously established predictors. The final cut-off level was chosen based on the most optimal separation between ITB and CD. In the next step, several logistic regression models were fitted. Due to the limited number of patients we included up to four risk factors in one model. The best model was chosen based on its prediction power and the Aikaike Information Criterion [25]. Finally, the odds computed with the selected logistic regression model were transformed into probabilities with 95% Confidence Intervals (CI) and the results were arranged in a risk matrix. The analyses were conducted with Predictive Analytics Software (Version 18.1; IBM, New York, USA).
Variables
Univariate analysis
Multivariate analysis
ITB
n/Total (%)
CD
n/Total (%)
P*
OR†
95%CI
P
Right inferior abdominal pain
15/28(54)
27/30(90)
0.002
0.10
0.02-0.51
0.005
Weight loss
27/37(73)
14/37(38)
0.002
8.6
2.1-35.6
0.003
Mucosal nodularity
17/31(55)
2/37(5)
<0.001
18.9
3.5-102.8
0.001
Multi-segment involvement
9/31(29)
27/37(73)
<0.001
0.17
0.05-0.58
0.005
*Chi-squareorFischer’sexacttests.
†OR (odds ratio)>1indicates increased odds for ITB at diagnosis; OR<1 indicates increased odds for CD at diagnosis. CI, Confidence Interval.
Table 1: Univariate and multiple logistic regression analysis of selected clinical and endoscopic disease characteristics at diagnosis in patients with Intestinal Tuberculosis (ITB) and Crohn’sDisease (CD). (InpartialreproducedfromLarssonGetal.WorldJGastroenterol.2014; 20:5 017- 5024, Table5.).
Ethics
The study was approved by the Ethical Committee of Sree Gokulam Medical College and Research Foundation, Trivandrum, India and by the Ethical Committee of the Norwegian South Eastern Regional Health Authority. Written informed consent was obtained after explaining the study to the participants in their preferred language.
Results
Demographic, clinical, endoscopic and histologic features
Thirty-eight ITB patients (median age 33 years (range 21-68), 22 men) and 37 CD patients (median age 33 years (range 18-76), 24 men) were included. The demographic, clinical and endoscopic features of both patient categories were presented in our previous report [2]. Upon histopathological examination of targeted intestinal biopsies, the granuloma detection rate in ITB was 10/30 (33%) and in CD 2/35 (6%), respectively. Caseous necrosis was not observed in any of the biopsies.
Biochemical features
Calprotectin and CRP were analysed in the ITB and CD patients at the time of diagnosis. A significantly higher median FC level (p=0.046) was observed in ITB (320μg/g, min-max: 0- 1800μg/g, inter-quartile range (IQR) 942) than in CD (133μg/g, min-max: 0-1000μg/g, IQR 274). Also, median SC and CRP levels were higher in ITB (SC 5.7μg/mL, min-max: 0.0- 18.0μg/mL, IQR 7.0; CRP 10.7mg/L, min-max: 0.2-70.5mg/L, IQR 37.6) than in CD (SC 4.0 μg/ mL, min-max: 0.1-30.0μg/mL, IQR 8.3; CRP 4.3 mg/L, min-max: 0.3- 49.8mg/L, IQR 12.7). However, the differences in SC and CRP levels between the groups did not reach statistical significance. In the CD cohort, we found a moderate association between the FC and CRP levels (rs = 0.40, p = 0.03), and no association between the SC and CRP levels or between the SC and FC levels. The associations between FC, SC and CRP in ITB were presented recently [17]. Calprotectin and CRP levels were not influenced by any of the endoscopic features recorded at the time of diagnosis (data not shown).
Probability of ITB or CD diagnosis
Of the four variables significantly associated with either ITB or CD diagnosis, we excluded the variable right inferior abdominal pain from further analyses because of the relatively low response numbers and because it strongly correlated with the variables multisegment involvement and weight loss (Table 1). To avoid further multicollinearity, CRP was also excluded from regression analyses because of the association with FC. SC was excluded because it did not differ significantly between the groups. Accordingly, four variables were significantly associated with ITB or CD and these were combined in a final visual risk matrix: weight loss, mucosal nodularity, multi-segment involvement and F-calprotectin = 200μg/g (Figure 1). The highest probability was computed for ITB patients with the combination of F-calprotectin = 200μg/g, mucosal nodularity, no multi-segment involvement and no weight loss. This risk factor profile was associated with a 91% probability of ITB diagnosis (95% CI: 85-98%). The lowest probability of ITB diagnosis was computed for patients with F-calprotectin < 200μg/g, weight loss, multi-segment involvement and no mucosal nodularity. This risk factor profile was associated with a 19% probability of ITB diagnosis (95% CI: 10-28%). Because the outcome of not having ITB was the diagnosis of CD, patients with the lowest probability of ITB diagnosis were most likely to have CD (81% probability). We observed that the probabilities of ITB or CD diagnosis were very similar regardless of the values for weight loss or multi-segment involvement (Figure 1) and therefore, these two variables did not improve our ability to differentiate between ITB and CD.
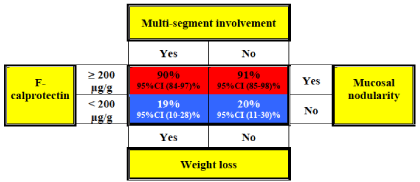
Figure 1: Risk Matrix: Diagnostic predictors of intestinal tuberculosis, Red
indicates a higher probability of intestinal tuberculosis at diagnosis, Blue
indicates a higher probability of Crohn’s Disease at diagnosis.
Discussion
In this multi-centre, population based observational cohort study we have demonstrated that the probability of ITB or CD diagnosis could be predicted by a risk matrix model. The visual matrix shows the probability of ITB or CD diagnosis given a specific combination of risk factors at diagnosis. The probability of ITB at diagnosis ranged from 19 to 91%, depending on the level of the risk factors. Similarly, the probability of CD at diagnosis ranged from 9 to 81%.
Univariate and multivariate analyses of mucosal nodularity, weight loss and multi-segment involvement showed significant differences between ITB and CD (Table 1). We expected that the combination of several criteria would increase the probability of the one or the other diagnosis. However, the transformation of odds to probabilities in the risk matrix revealed that only the levels of mucosal nodularity and f-calprotectin influenced the final diagnosis. Also, weight loss and multi-segment involvement, or the absence of such, hardly influenced the probability of ITB or CD (Figure 1). Furthermore, the variable weight loss depended on the patients’ availability to record their body weight, an objective measure practically inaccessible to most people. Moreover, the recording of multi-segment involvement could potentially be affected by inter-operator variability. Hence, excluding the variables weight loss and multisegment involvement from the probability matrix would not only simplify the assessment, but could also discard the effect of bias on these variables.
The matrix showed that the evaluation of f-calprotectin and mucosal nodularity would be sufficient to make the most probable diagnosis. For patients who do not fit in the model mucosal nodularity could be regarded as the decisive variable as it was superior to the other predictors (Figure 1). Previously, FC has been well described in active CD, with levels ranging from moderately elevated to very high, depending on the severity and location of disease [26,27]. In this study, although the severity of disease observed in the patient categories was comparable, a significantly higher median FC level was detected in ITB than in CD. However, a significantly higher number of lesions and more widespread intestinal involvement were detected upon endoscopy in CD compared to ITB [2]. Hence, active intestinal inflammation seemed to be a stronger trigger of FC release than extensive intestinal involvement. Mycobacteria typically recruit and invade neutrophils, macrophages and monocytes during infection [9], these are important sources of calprotectin secretion [28]. Although granulocytes become activated and secrete calprotectin during CD flares [29], the immune response in CD is primarily T-cell mediated. In fact, greater secretion of neutrophil attractants has been demonstrated in ITB compared with CD [10]. These immunological dissimilarities could further explain the differences in FC levels recorded between ITB and CD. Certainly, CD patients with severe inflammation could have much higher FC levels than the median FC level recorded in our CD cohort. However, severe disease would typically include the colon and hence, multi-segment involvement and the absence of mucosal nodularity would favour a diagnosis of CD, judging from the visual matrix. Therefore, the risk matrix also could be considered in the differential assessment of patients with severe disease. The elevated FC levels found in ITB were higher than what have previously been reported in other bacterial gastrointestinal infections [30, 31]. However, very high SC and CRP levels have been demonstrated in acute bacterial infections [28], opposed to the moderately elevated levels detected in our ITB patients suggestive of chronic inflammation. Still, the median SC and CRP levels were higher in ITB than in CD, thus reflecting mild systemic inflammation. This was further substantiated by the finding of a moderate association between SC and CRP levels in the ITB group. The low median SC level detected in our CD group was somewhat similar to the levels seen in patients with functional gastrointestinal disorders [17]. Future studies should investigate whether SC determination adds any useful information to the diagnostic work-up and follow-up in ITB and CD.
Certain “gold standard” diagnostic methods were unavailable because of the regional scarcity in economical and personnel resources, including computer tomography and magnetic resonance enterography. Furthermore, Mantoux tuberculin skin-testing and interferon-γ release assays were not used. These tests have proven unable to differentiate latent (Mycobacterium tuberculosis (M.tb)) infection from active TB in endemic areas, as positive results simply report the presence of specific T cell responses irrespective of the underlying clinical condition [3-32]. Also, although positive acidfast staining and/or culturing of M.tb from intestinal biopsies are predictive of TB, these methods were not applied, primarily due to their low yield demonstrated in previous studies [1-32]. Additionally, it may be unfavorable to leave a patient untreated pending the result of a slow growing M.tb culture. The lack of microbiological testing is a challenge in most TB endemic regions and was agreeably a limitation in this study. However, our intention was to evaluate the ITB and CD patients’ biochemical, clinical and endoscopic features within the frame of current regional clinical practice. Several authors have concluded that the evaluation of response to empiric ATT is an acceptable method in the diagnostic work-up of ITB in economically deprived TB endemic areas [3-12]. Although debateable, because of limited resources, categorizing a positive treatment response as confirmatory of ITB remains the only available diagnostic method for most cases worldwide. Moreover, TB control relies on passive case finding among individuals self-presenting to health care facilities. For many, the costs of repeated visits to health centres for recurrent diagnostic tests or imaging procedures are prohibitive, and patient dropout is a significant problem [33]. After all, the cornerstone of TB control is prompt treatment and this may be facilitated by the application of rapid diagnostic tests. In areas with a scarcity of “gold standard” diagnostics, tools involving POC devices could become important assets in routine clinical practice [33,34]. Future research on ITB in economically deprived areas should aspire towards collaboration with high tech medical institutions to further evaluate the diagnostic potential of these devices. Because ITB and CD may be of heterogeneous phenotypes, methods to predict disease severity and to differentiate the one disease from the other should include a combination of risk factors. However, the list of risk factors included in our model is not exhaustive, and the matrix needs to be validated in new studies to assess whether rearrangement of variables or fineCertain “gold standard” diagnostic methods were unavailable because of the regional scarcity in economical and personnel resources, including computer tomography and magnetic resonance enterography. Furthermore, Mantoux tuberculin skin-testing and interferon-γ release assays were not used. These tests have proven unable to differentiate latent (Mycobacterium tuberculosis (M.tb)) infection from active TB in endemic areas, as positive results simply report the presence of specific T cell responses irrespective of the underlying clinical condition [3-32]. Also, although positive acidfast staining and/or culturing of M.tb from intestinal biopsies are predictive of TB, these methods were not applied, primarily due to their low yield demonstrated in previous studies [1-32]. Additionally, it may be unfavorable to leave a patient untreated pending the result of a slow growing M.tb culture. The lack of microbiological testing is a challenge in most TB endemic regions and was agreeably a limitation in this study. However, our intention was to evaluate the ITB and CD patients’ biochemical, clinical and endoscopic features within the frame of current regional clinical practice. Several authors have concluded that the evaluation of response to empiric ATT is an acceptable method in the diagnostic work-up of ITB in economically deprived TB endemic areas [3-12]. Although debateable, because of limited resources, categorizing a positive treatment response as confirmatory of ITB remains the only available diagnostic method for most cases worldwide. Moreover, TB control relies on passive case finding among individuals self-presenting to health care facilities. For many, the costs of repeated visits to health centres for recurrent diagnostic tests or imaging procedures are prohibitive, and patient dropout is a significant problem [33]. After all, the cornerstone of TB control is prompt treatment and this may be facilitated by the application of rapid diagnostic tests. In areas with a scarcity of “gold standard” diagnostics, tools involving POC devices could become important assets in routine clinical practice [33,34]. Future research on ITB in economically deprived areas should aspire towards collaboration with high tech medical institutions to further evaluate the diagnostic potential of these devices. Because ITB and CD may be of heterogeneous phenotypes, methods to predict disease severity and to differentiate the one disease from the other should include a combination of risk factors. However, the list of risk factors included in our model is not exhaustive, and the matrix needs to be validated in new studies to assess whether rearrangement of variables or finetuning would be necessary. Also, the predictive values in the model should be confirmed in new studies to diminish resubstitution bias [35]. The study was limited by the sample size of altogether 75 patients. Only crude statistical methods were applied to assess the associations between the continuous variables and we are aware of the reduced statistical power to reveal smaller but perhaps clinically relevant differences between the groups. Future research should investigate these associations further. The population based, multicentre, prospective study design was advantageous and diminished selection bias.
Conclusion
By combining FC measurements with clinical and endoscopic risk factors in a visual risk matrix model, we present the probability of ITB based on selected variables measured at diagnosis. Our model could be developed as a tool to distinguish between ITB and CD in everyday clinical practice, especially in economically challenged high burden TB areas.
Acknowledgement
Dr. G Larsson would like to thank the staff at the Population Health and Research Institute in Trivandrum, India, with special regards to Mrs. Suja, for handling and preparing patient samples and filing the data. Special thanks also to the investigator at Coimbatore centre (Dr. VG Mohan Prasad) for recruiting patients. Calprotectin analyses were performed with kits provided without cost by Bühlmann Laboratories, AG (Basel, Switzerland). Dr. A Røseth kindly advised on the analysis of calprotectin. Thanks also to Dr. B Holm and Mrs. AM Tangen at Lovisenberg Diaconal Hospital for allowing the initiation and financial support of this study. Dr. PC Klepp is acknowledged for proofreading and thorough support during the study.
References
- Almadi MA, Ghosh S, Aljebreen AM. Differentiating intestinal tuberculosis from Crohn's disease: a diagnostic challenge. Am J Gastroenterol 2009 Apr; 104:1003- 12.
- Larsson G, Shenoy T, Ramasubramanian R, Balakumaran LK, Smastuen MC, Bjune GA, et al. Routine diagnosis of intestinal tuberculosis and Crohn's disease in Southern India. World J Gastroenterol 2014 May 7; 20:5017-24.
- Makharia GK, Srivastava S, Das P, Goswami P, Singh U, Tripathi M, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol 2010 Mar; 105:642-51.
- Li X, Liu X, Zou Y, Ouyang C, Wu X, Zhou M, et al. Predictors of clinical and endoscopic findings in differentiating Crohn's disease from intestinal tuberculosis. Dig Dis Sci 2011 Jan; 56:188-96.
- Sibartie V, Kirwan WO, O'Mahony S, Stack W, and Shanahan F. Intestinal tuberculosis mimicking Crohn's disease: lessons relearned in a new era. Eur J Gastroenterol Hepatol 2007 Apr; 19:347-9.
- Singh DD, Vogel M, Muller-Stover I, El ST, Winzer M, Gobels S, et al. TB or not TB? Difficulties in the diagnosis of tuberculosis in HIV-negative immigrants to Germany. Eur J Med Res 2011 Sep 12; 16:381-4.
- Mukewar S, Mukewar S, Ravi R, Prasad A, Dua S. Colon tuberculosis: endoscopic features and prospective endoscopic follow-up after anti-tuberculosis treatment. Clin Transl Gastroenterol 2012; 3:e24.
- Alvares JF, Devarbhavi H, Makhija P, Rao S, Kottoor R. Clinical, colonoscopic, and histological profile of colonic tuberculosis in a tertiary hospital. Endoscopy 2005 Apr; 37:351-6.
- Kirsch R, Pentecost M, Hall PM, Epstein DP, Watermeyer G, Friederich PW. Role of colonoscopic biopsy in distinguishing between Crohn's disease and intestinal tuberculosis. J Clin Pathol 2006 Aug; 59:840-4.
- Pugazhendhi S, Jayakanthan K, Pulimood AB, Ramakrishna BS. Cytokine gene expression in intestinal tuberculosis and Crohn's disease. Int J Tuberc Lung Dis 2013 May; 17:662-8.
- Ye BD, Yang SK, Kim D, Shim TS, Kim SH, Kim MN, et al. Diagnostic sensitivity of culture and drug resistance patterns in Korean patients with intestinal tuberculosis. Int J Tuberc Lung Dis 2012 Jun; 16:799-804.
- Epstein D, Watermeyer G, Kirsch R. Review article: the diagnosis and management of Crohn's disease in populations with high-risk rates for tuberculosis. Aliment Pharmacol Ther 2007 Jun 15; 25(12):1373-88.14
- Tibble J, Teahon K, Thjodleifsson B, Roseth A, Sigthorsson G, Bridger S, et al. A simple method for assessing intestinal inflammation in Crohn's disease. Gut 2000 Oct; 47:506-13.
- Labaere D, Smismans A, Van OA, Christiaens P, D'Haens G, Moons V, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J 2014 Feb; 2:30-7.
- Henriksen M, Jahnsen J, Lygren I, Stray N, Sauar J, Vatn MH, et al. C-reactive protein: a predictive factor and marker of inflammation in inflammatory bowel disease. Results from a prospective population-based study. Gut 2008 Nov; 57:1518-23.
- Meuwis MA, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Piver E, et al. Serum calprotectin as a biomarker for Crohn's disease. J Crohns Colitis 2013 Dec 15; 7:e678-e683.
- Larsson G, Shenoy K, Ramasubramanian R, Thayumanavan L, Balakumaran L, Bjune G, et al. Faecal calprotectin levels differentiate intestinal from pulmonary tuberculosis: an observational study from Southern India. United European Gastroenterology Journal October 2014 2: 397–405.
- Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De BG, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003 Jun; 24:987-1003.
- Solberg IC, Cvancarova M, Vatn MH, Moum B. Risk matrix for prediction of advanced disease in a population-based study of patients with Crohn's Disease (the IBSEN Study). Inflamm Bowel Dis 2014 Jan;20:608.
- Patel N, Amarapurkar D, Agal S, Baijal R, Kulshrestha P, Pramanik S, et al. Gastrointestinal luminal tuberculosis: establishing the diagnosis. J Gastroenterol Hepatol 2004 Nov; 19:1240-6.
- Ouyang Q, Tandon R, Goh KL, Pan GZ, Fock KM, Fiocchi C, et al. Management consensus of inflammatory bowel disease for the Asia-Pacific region. J Gastroenterol Hepatol 2006 Dec; 21:1772-82.
- D'Haens G, Ferrante M, Vermeire S, Baert F, Noman M, Moortgat L, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012 Dec; 18:2218-24.
- Pavlidis P, Chedgy FJ, Tibble JA. Diagnostic accuracy and clinical application of faecal calprotectin in adult patients presenting with gastrointestinal symptoms in primary care. Scand J Gastroenterol 2013 Sep; 48:1048-54.
- Lobaton T, Lopez-Garcia A, Rodriguez-Moranta F, Ruiz A, Rodriguez L, Guardiola J. A new rapid test for fecal calprotectin predicts endoscopic remission and postoperative recurrence in Crohn's disease. J Crohns Colitis 2013 Dec 15; 7:e641-e651.
- Akaike H. [Data analysis by statistical models]. No To Hattatsu 1992 Mar; 24:127- 33.15
- Schoepfer AM, Beglinger C, Straumann A, Trummler M, Vavricka SR, Bruegger LE, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn's disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010 Jan; 105:162-9.
- Jones J, Loftus EV, Jr., Panaccione R, Chen LS, Peterson S, McConnell J, et al. Relationships between disease activity and serum and fecal biomarkers in patients with Crohn's disease. Clin Gastroenterol Hepatol 2008 Nov; 6:1218-24.
- Johne B, Fagerhol MK, Lyberg T, Prydz H, Brandtzaeg P, Naess-Andresen CF, et al. Functional and clinical aspects of the myelomonocyte protein calprotectin. Mol Pathol 1997 Jun; 50:113-23.
- Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol 1999 Jan; 34:50-4.
- Shastri YM, Bergis D, Povse N, Schafer V, Shastri S, Weindel M, et al. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am J Med 2008 Dec; 121:1099-106.
- Weh J, Antoni C, Weiss C, Findeisen P, Ebert M, Bocker U. Discriminatory potential of C-reactive protein, cytokines, and fecal markers in infectious gastroenteritis in adults. Diagn Microbiol Infect Dis 2013 Sep; 77:79-84.
- Amarapurkar DN, Patel ND, Rane PS. Diagnosis of Crohn's disease in India where tuberculosis is widely prevalent. World J Gastroenterol 2008 Feb 7; 14:741-6.
- Parsons LM, Somoskovi A, Gutierrez C, Lee E, Paramasivan CN, Abimiku A, et al. Laboratory diagnosis of tuberculosis in resource-poor countries: challenges and opportunities. Clin Microbiol Rev 2011 Apr; 24:314-50.
- Nakiyingi L, Bwanika JM, Kirenga B, Nakanjako D, Katabira C, Lubega G, et al. Clinical predictors and accuracy of empiric tuberculosis treatment among sputum smear-negative HIV-infected adult TB suspects in Uganda. PLoS One 2013; 8:e74023.
- Kerr KF, Meisner A, Thiessen-Philbrook H, Coca SG, Parikh CR. RiGoR: reporting guidelines to address common sources of bias in risk model development. Biomark Res 2015; 3.