
Review Article
Austin J Gastroenterol. 2015; 2(5): 1054.
Ulcerative Colitis Associated with Cholangiocellular Carcinoma: A Case Report and Literature Review
Fang Y¹, Lei L¹*, Shen X¹, Wang J¹ and Sheng R²
¹Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, China
²Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, China
*Corresponding author: Lei Li, Department of Gastroenterology and Hepatology, Zhongshan Hospital, Fudan University, China
Received: July 07, 2015; Accepted: September 09, 2015; Published: September 11, 2015
Abstract
To investigate the possibility for Ulcerative Colitis (UC) to progress to cholangiocellular carcinoma (CCC), clinical data of a UC patient with concomitant CCC were analyzed and the relevant literature was reviewed. The patient was diagnosed with UC 14 years prior and had been treated with sulfasalazine. Her UC symptoms were alleviated after administration of sulfasalazine, but liver function was repeatedly found to be abnormal. She was diagnosed with CCC by ultrasound and magnetic resonance imaging. A review of the literature suggests that UC is easily associated with concomitant primary sclerosing cholangitis, which progresses to CCC in 11% of cases. Clinicians should be aware that UC may progress into CCC. UC patients with repeated abnormal liver function, especially those who have serum alkaline phosphatase elevation, should undergo relevant examinations to clarify the diagnosis.
Keywords: Ulcerative colitis; Primary sclerosing cholangitis; Cholangiocellular carcinoma
Introduction
Primary Sclerosing Cholangitis (PSC) is the most common hepatobiliary complication of Ulcerative Colitis (UC). The majority of PSC cases ultimately progress to end-stage liver disease, and ~11% are associated with concomitant Cholangiocellular Carcinoma (CCC). This study provides a clinical analysis of a UC patient with concomitant CCC and reviews the relevant literature to raise awareness of the possibility of progression of UC to CCC.
Clinical Data
General data
The patient was a 24-year-old woman who was admitted to our hospital because of repeated abnormal liver function over a period of 14 years and upper abdominal pain for >1 month. She started taking mesalazine after she was diagnosed with UC in 1999. Several follow-up examinations of liver function found elevation of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), and γ-glutamyl transpeptidase (GGT). In particular, ALP and GGT were elevated to 2–3-fold of the Upper Limit of Normal (ULN). During follow-up, the patient had no discomfort such as bloating or abdominal pain; no nausea, vomiting or anorexia; and no fever, rash or xanthochromia of the skin and sclera. No attention was paid and no further diagnosis or treatment was received. In 2009, her UC was well controlled and the dose of mesalamine was therefore gradually reduced to withdrawal. Six months after withdrawal of mesalamine, liver function tests showed elevated liver enzymes, mainly ALP and GGT that were 1–2- fold of ULN. The patient visited the hospital but liver biopsy showed no obvious abnormalities. She was given intermittent, symptomatic treatment of the liver. In February 2015, she had upper abdominal pain after meals, with no obvious predisposing causes. The patient denied acid reflux, belching, nausea, vomiting, diarrhea, fever, rash, jaundice, joint pain and other discomforts. She visited a local hospital and liver function tests showed: total bilirubin 21 μmol/L, conjugated bilirubin 14 μmol/L, albumin 42 g/L, ALT 117 IU/L, AST 131 IU/L, and ALP 962 IU/L, and she was negative for hepatitis A, B, C and E virus markers. Gastroscopy showed superficial gastritis, polypoid bulge, and erosion in the gastric antrum, and cardiac polyp (probably inflammatory). Gastric antral mucosal biopsy showed severe active chronic superficial inflammation in the mucosa with erosion. Abdominal ultrasound showed hepatomegaly, diffuse liver lesion, and hypoechoic mass in the left hepatic lobe, and gallbladder wall edema. Abdominal contrast-enhanced CT showed focal liver lesions, suspicious for neoplastic lesions, probably atypical hemangioma or bile-duct-derived tumors. To clarify the nature of focal liver lesions, the patient was admitted to the Department of Gastroenterology in our hospital. Since disease onset, she remained in a clear state of consciousness with good mental health, good appetite, sufficient sleep, normal urination and defecation, and no significant weight change. She had a 14-year history of UC and took mesalazine previously. Six years prior to admission, use of mesalazine was gradually reduced to withdrawal because of good disease control, and she was still in remission. She underwent an appendectomy at Beijing Children’s Hospital in 1999, with a history of allergy to cephalosporins. She denied a history of hepatitis, smoking, alcohol drinking, or toxicant exposure. She was unmarried and had not given birth, and had a normal menstrual cycle.
Physical examination
The patient had a body temperature of 36.8°C, pulse rate of 78 beats/min, respiratory rate of 18 beats/min, and blood pressure of 110/78 mmHg. She was clearly conscious, mentally normal, physically active, and cooperative upon examination. Her skin and sclera showed xanthochromia and there were no hemorrhagic spots, liver palms, or spider angioma on the skin. She had no enlargement of systemic superficial lymph nodes. Her percussion and breath sounds of both lungs were clear. She had no precordial bulge, and the border of cardiac dullness was within normal limits (heart rate 78 beats/ min). Her heart rhythm was regular and no pathological murmurs were auscultated in auscultatory valve areas. Her abdomen was flat with a 5-cm score in the center of the abdomen. No subcutaneous varicose veins were observed in the abdominal wall. She had a soft abdomen, with right upper quadrant tenderness but no rebound tenderness, muscle tension or mass. Her liver was palpable with one finger under the costal margins, while the spleen was not palpable. Her renal and hepatic regions were not sensitive to percussion. Shifting dullness was negative. Bowel sound frequency was 3 times/ min. Neurological examination produced negative results. No pitting edema was observed in either lower extremity.
Laboratory tests
Blood: erythrocyte count 3.60 × 1012/L, hemoglobin 104 g/L, platelet count 501 × 109/L, leukocyte count 9.67 × 109/L, percentage of neutrophils 71.3%, percentage of lymphocytes 16.2%; erythrocyte sedimentation rate: >120 mm/H. Urine: bilirubin ++, without any other abnormalities; stool: normal; fecal occult blood: negative. Liver function: total bilirubin 64.4 μmol/L, direct bilirubin 59.7 μmol/L, albumin 33 g/L, globulin 38 g/L, ALT 55 U/L, AST 61 U/L, ALP 561 U/L, GGT 382 U/L, lactate dehydrogenase 201 U/L, cholinesterase 4132 U/L, prealbumin 0.12 g/L; kidney function: urea 1.8 mmol/L, creatinine 39 μmol/L, uric acid 139 μmol/L. Coagulation: prothrombin time 12.2 s, international normalized ratio 1.05, activated partial thromboplastin time 36.5 s, D-dimer 2.17 mg/L. Tumor markers: carbohydrate antigen (CA)125 451.3 IU/mL; a fetoprotein, carcinoembryonic antigen, CA199 within the normal range. Ferritin >2 μg/mL electrolyte and autoantibodies within the normal range.
Auxiliary examination
Upper abdominal contrast-enhanced magnetic resonance imaging (Figure 1) revealed space-occupying lesions in the left hepatic lobe, with a tubercle in the caudal lobe involving blood vessels in the left lobe, suspicious for atypical hemangioma or bile duct Malignant Tumor (MT); other features included mild porta hepatis lymphadenopathy, and edema and thickening of the gallbladder. Contrast-enhanced ultrasound for liver imaging showed multiple solid lesions in the left lobe, highly suspicious for MT (cholangiocytederived); embolization in the left portal vein; and porta hepatis lymphadenopathy. Computed Tomographic Angiography (CTA) of the hepatic artery and computed tomography venography (CTV) of the portal vein, hepatic vein and inferior vena cava revealed invasion of the left hepatic artery, left and middle hepatic veins, and left portal vein. Nuclear medicine imaging of systemic cancer by Positron Emission Tomography (PET)/CT indicated that the patient was highly suspicious for MT in the left lobe with intrahepatic metastasis, and porta hepatis, retroperitoneal and thoracic lymph node metastases, as well as abdominal and pelvic implantation metastases, with a small amount of peritoneal fluid.
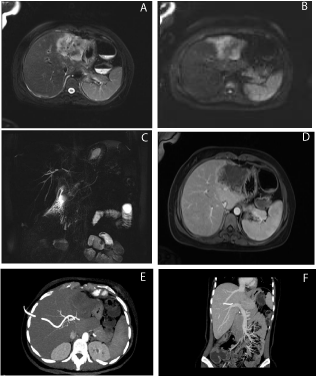
Figure 1: Large liver lesion in the left hepatic lobe with unclear boundary, approximately 7.1×5.1 cm; heterogeneous high signal on T2-weighted imaging (A) and
high signal on diffusion-weighted imaging (B); MRCP revealed irregular intrahepatic bile duct stricture (C); contrast-enhanced arterial phase lesions presented with
peripheral irregular enhancement (D); CTA of the hepatic artery and CTV of the portal vein, hepatic vein and hepatic artery indicated invasion of the left hepatic
artery, left and middle hepatic veins, and left hepatic portal vein, with less clear boundaries (E,F).
Diagnosis and treatment
The patient presented with progressive jaundice after admission. She had Percutaneous Transhepatic Cholangial Drainage (PTCD) on March 17, 2015. Intraoperative cholangiography revealed slight right intrahepatic bile duct dilatation and irregular right hepatic duct stricture. Jaundice was alleviated following PTCD.
Discussion
Inflammatory Bowel Disease (IBD) is an immune-mediated and non-specific inflammatory condition of the gastrointestinal tract characterized by repeated recurrence and chronicity, and includes UC and Crohn’s disease. It is reported that 21–47% of IBD cases are associated with extraintestinal manifestations [1,2]. The extraintestinal manifestations involving the skin, eyes, and joints are often present in parallel with the disease activity of IBD, while hepatobiliary system diseases such as PSC, small-duct PSC, primary biliary cirrhosis, non-alcoholic fatty liver diseases, and cholelithiasis are inconsistent with IBD progression but generally occur independent of intestinal inflammation. PSC is an unexplained chronic cholestatic liver disease characterized by progressive inflammation, fibrosis and multiple stenoses of the intrahepatic and extrahepatic bile duct systems. PSC is currently well recognized as the most common hepatobiliary complication of IBD. The relationship between PSC and IBD has been reported mainly in Western countries. Up to 80% of PSC cases are associated with IBD in Northern Europe and the United States. The incidence of IBD–PSC is 50% in Southern Europe and 35% in Asia, as is reported mainly in Japan and Korea. Few studies have been reported in China, and all recent reports on the incidence of IBD-PSC were single-center studies with small samples. Despite large differences in the results, the incidence of IBD-PSC is significantly lower in China than in Europe and the United States [3,4]. In other countries, 1.4–7.5% of IBD patients develop concomitant PSC during disease progression, with a UC incidence of 2.0–7.5% and a CD incidence of 1.4–3.4% [5]. Similar results have been obtain by recent studies in China [6].
The pathogenesis of PSC–IBD remains unclear. From the viewpoint of microbiology, IBD is diagnosed when intestinalmucosal barrier permeability increases, so that bacterial endotoxins and toxic cholic acids are increased in the blood and then transported to the liver to activate Kupffer cells and increase inflammatory mediators (e.g., tumor necrosis factor), causing bile duct damage and hyperplasia resembling the pathological changes in PSC. This point of view is supported by the finding of intestinal bacilli in the portal veins of an animal model, although the exact pathogenesis remains unclear [7].
Significantly different clinical manifestations can be found in PSC, which may have no obvious symptoms, or present with recurrent episodes of jaundice, fatigue, anorexia, weight loss, abdominal pain, itching, hepatosplenomegaly, ascites, fever and diarrhea. Most PSC patients are asymptomatic at diagnosis, but only have serum ALP elevation or abnormalities of other biochemical indices of hepatic function. Thus, PSC is easily overlooked, leading to clinical misdiagnosis. Therefore, it has been suggested that attention should be paid to IBD patients with persistent abnormalities of liver function, especially who have persistent ALP elevation, for the possibility of concomitant PSC. Current diagnosis of PSC is mainly based on imaging. Although Endoscopic Retrograde Cholangio Pancreatography (ERCP) is the gold standard [8], it is an invasive examination and thus can lead to serious complications. Owing to its non-invasiveness and ease of operation, Magnetic Resonance Cholangiography (MRCP) is increasingly used for diagnosis of PSC, with a sensitivity of 80% and specificity of 90% [9]. The typical pathological feature of PSC is the “onion-skin” type bile duct fibrosis. This pathological change can be used as the basis for diagnosis and determination of lesion severity. However, such a pathological feature applies to only 10% of PSC patients, and thus has limited diagnostic value. Research has shown that liver biopsy can provide no more diagnostic information for IBD patients after being examined by cholangiography [10]. Therefore, liver biopsy is not recommended for patients with typical lesions as revealed by cholangiography [11]. Liver biopsy needs to be further performed only when the diagnosis by ERCP is suspicious. The patient reported in this study had a 14-year history of UC and previously had taken mesalazine for a long time. In the course of the disease, her liver function was repeatedly found to be abnormal, with ALP and GGT elevation being the most common abnormalities but with no obvious clinical manifestations. She was misdiagnosed with mesalamine-induced liver damage, without further related checks. After recent admission to our hospital, MRCP showed irregular intrahepatic biliary stricture (Figure. 1E and 1F). Cholangiography during PTCD showed right intrahepatic bile duct dilatation and irregular right hepatic duct stricture. No abnormality was found by liver biopsy; however, as described above, liver biopsy has limitations and therefore, the patient was still considered to have UC with concomitant development of PSC.
PSC has a poor prognosis, with an average survival period of 9.6–18 years. The risk ratio of PSC associated with various malignant tumors is 13–14%; most commonly CCC (11%) followed by colorectal carcinoma (9%). Other concomitant malignancies include hepatocellular carcinoma, gallbladder carcinoma and pancreatic carcinoma [9]. Progression of PSC into CCC occurs mainly in patients with a >15-year history of IBD, or patients who have pancolitis, irrespective of disease severity [12]. CCC–PSC is present in adolescents as well as adults [13]. After admission to our hospital, MRCP, contrast-enhanced ultrasound and PET–CT all prompted bile-duct-derived malignancy in our patient. Hence, she was considered to have concomitant PSC in the course of UC, which further progressed to CCC. Since the patient already had distant metastases, she was diagnosed with end-stage CCC.
Presently, there are still no drugs that can alter disease progression or the natural course of PSC. Studies have shown that low-dose ursodeoxycholic acid (UDCA; 13–15 mg/kg/day) has a positive role in improving liver function in patients with PSC, while high-dose UDCA (28–30 mg/kg/day) does not add any benefits but increases the risk to patients [14]. Recent clinical follow-up studies with large samples have indicated that although UDCA can improve the biochemical indices for PSC patients, it has no effect on liver histology and no significant impact on mortality, need for liver transplantation, and prognosis of CCC [15]. Despite that endoscopic intervention and surgical procedures to some extent alleviate biliary obstruction and reduce liver damage, liver transplantation is still preferred for end-stage PSC or CCC–PSC. Liver transplantation is the only effective treatment, with 5- and 10-year survival rates of 85% and 70% post-transplantation, respectively. However, our patient already had distant metastases and liver transplantation would have had little effect, and interventional therapy was applied only to alleviate her symptoms.
While there are a number of reports on PSC–UC in other countries, few studies have been reported in China. Thus far, there has been no report of PSC–UC with progression to CCC. Clinicians should be aware of the disease and pay attention to UC patients who repeatedly have abnormal liver function test results. In particular, for patients who have persistent ALP elevation, MRCP or ERCP should be used to clarify the diagnosis and provide intervention as early as possible.
References
- Lichtenstein DR. Hepatobiliary complications of inflammatory bowel disease. Curr Gastroenterol Rep. 2011; 13: 495-505.
- Bernstein CN, Blanchard JF, Rawsthorne P, The prevalence of extraintestinal diseases in inflammatory bowel disease: a population-based study. Am J Gastroenterol. 2001; 96: 1116-1122.
- Luo KF, Su X, Zhang XM, Li W. Primary sclerosing cholangitis: A clinical analysis of 26 cases . Academic Journal of Chinese PLA Medical School. 2014; 35: 404-407.
- Liu X, Du ZG, Jia JD. Detection rate of primary sclerosing cholangitis in 160 cases of ulcerative colitis. Chinese Journal of Hepatology. 2005; 13: 614.
- Boonstra K, van Erpecum KJ, van Nieuwkerk KMJ. Primary sclerosing cholangitis is associated with a distinct phenotype of inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18: 2270-2276.
- Zhang KG, Yin BS, Yang RS, Wang SM, Zheng BH, Jia Y, et al. Study of clinical features in patients with ulcerative colitis accompanied by primary sclerosing cholangitis. Journal of Rare and Uncommon Diseases. 2001; 8: 7-8.
- Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol. 2009; 31: 383-397.
- Lee YM, Kaplan MM. Primary sclerosing cholangitis. N Engl J Med. 1995; 332: 924-933.
- Lutz H, Trautwein C, Tischendorf JW. Primary sclerosing cholangitis: diagnosis and treatment. Dtsch Arztebl Int. 2013; 110: 867-874.
- Burak KW, Angulo P, Lindor KD. Is there a role for liver biopsy in primary sclerosing cholangitis?. Am J Gastroenterol. 2003; 98: 1155-1158.
- Chapman R, Fevery J, Kalloo A, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010; 51: 660-678.
- Moreno Luna LE, Gores GJ. Advances in the diagnosis of cholangiocarcinoma in patients with primary sclerosing cholangitis. Liver transplantation. 2006; 12: S15-S19.
- Liu R, Cox K, Guthery SL, et al. Cholangiocarcinoma and high-grade dysplasia in young patients with primary sclerosing cholangitis. Dig Dis Sci. 2014; 59: 2320-2324.
- Triantos CK, Koukias N, Nikolopoulou V. Ursodeoxycholic acid in primary sclerosing cholangitis. Aliment Pharmacol Ther. 2012; 35: 622-623.
- Triantos CK, Koukias NM, Nikolopoulou VN. Meta-analysis: ursodeoxycholic acid for primary sclerosing cholangitis. Aliment Pharmacol Ther. 2011; 34: 901-910.