
Special Article - Pancreatic Cancer
Austin J Gastroenterol. 2016; 3(2): 1064.
Newer Immunotherapeutic Approaches in Managing Metastatic Pancreatic Cancer
Arlen M¹*, Arlen P³, Coppa G¹, Crawford J¹, Dubukovskiy A², Saric O³, Conte C¹ and Molmenti E¹
¹Division of Surgical Oncology, NorthShore University Health System, USA
²Division of Pathology, NorthShore University Health System, USA
³Precision Biologics, Northwell Health System Hofstra College of Medicine, USA
*Corresponding author: Arlen M, Division of Surgical Oncology, NorthShore University Health System, USA
Received: May 23, 2016; Accepted: August 26, 2016; Published: August 29, 2016
Abstract
Pancreas Cancer remains one of the more difficult lesions to manage whether at the time of diagnosis or when seen for recurrent disease. When the tumor is operable by one of several surgical procedures depending on site of origin, survival rarely exceeds 10% at the 2 year level post op. When initially diagnosed with a metastatic lesion, survival of the patient rarely reaches 1 year. Chemotherapy becomes the standard method of treatment. When Gemcitabine is utilized the survival is found to be in the range of 5 months and when this is followed by Abraxane, an additional 7-10 wks can be expected.
In order to improve survival beyond that achieved by chemotherapy alone, immunotherapeutic agents are being introduced, with the hope of enhancing the overall survival rate. Many immunogenic targets have been defined, but those offering the best opportunity for accomplishing the needed response are proteins (TAA’s) expressed by the tumor that are immunogenic and specifically characterize that lesion without cross reactivity to normal tissue.
We have found in our studies of colorectal carcinoma that several tumor associated antigens are present and that one in particular, the post translational modification of MUC5ac is highly expressed in many cases of pancreatic cancer. This protein is present mainly tumors of the colon and pancreas but at levels too low to be recognized by the host immune system. After isolating this TAA and measuring levels of its expression by the tumor, few lesions contain more than 25-50 ugms. A detailed study of levels necessary to induce the proper immune response has been shown to be between 500 and 1000 ugms. We have also looked at mechanisms by which TAA, when delivered at proper levels produce immune suppression of the lesion. The mechanism has been shown to primarily be IgG1 expression by the B cells and that the cytotoxic T cells do not play a major role. The monoclonals do not directly affect the tumor by rather function through ADCC (antibody dependent cell cytotoxicity). In the present paper discussing our clinical trials, patients entered have failed all therapeutic approaches, have been shown to express the proper target antigen and as such receive 400 mg. antibody IV q 2 wks. The nature of the tumor antigen, development of the monoclonal system employed and status of the ongoing FDA trial is described.
Keywords: Pancreatic cancer; Tumor; Patients
Introduction
Among the various solid tumor malignancies, one of the more aggressive lesions, one that exhibits a high mortality rate virtually from its earliest stages of inception is the pancreatic adenocarcinoma [1]. This is based on the fact that among those primary lesions to be encountered and evaluated during early stages of development, that metastasis will probably be detected in most of the patients [2]. Survival, for such lesions is for the most part based on progression of disease seen months after diagnosis rather than the longer periods seen with other GI malignancies. The end stage for this form of cancer occurs rapidly even though numerous therapeutic approaches have been applied to control the primary lesion and any existing metastatic growths. Should one detect the primary early enough in its clinical onset, so that resection of tumor is feasible, at the end of a two year period post surgery, roughly 10% of patients will have survived [3]. As such diagnosis usually is achieved late in the clinical course of disease.
Early recognition of tumor presence is usually not accomplished in spite of detecting minor complaints with minimal symptoms related to the appearance of the pancreatic cancer. At the present time the use of blood tests defining serum markers specific for pancreatic cancer are not that effective [4,5,6].
Should one examine the histologic findings that define the early onset of this disease, it will be noted that the initial transformation to malignancy arises within the ductal mucosa of the pancreas in a pattern similar to what is seen with ductal carcinoma in situ (DCIS) of the breast [7] (Figure 1). The genetic alterations occurring within the mucosal cell occurs over a 15-20 year period of time until the insitu premalignant cells show signs of ductal invasion to then present as an early lesion within pancreatic parenchyma [8].
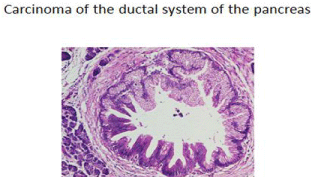
Figure 1: Early Intraductal alterations representing in-situ Ca.
Several patterns of clinical expression can be noted depending on where the lesion arises within the ductal system and as such where the lesion itself is defined with relationship to head, body or tail of the gland when first detected. The pancreas as an organ lies in the upper abdomen, the head presenting within the duodenal sweep and the body extending across the upper abdomen (Figure 2). The distal tail portion extends to the splenic hilum [9].
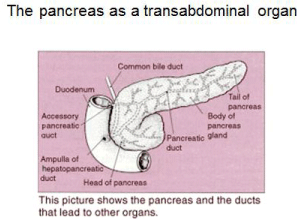
Figure 2: The position in which the tumor arises with regard to pancreas
and surrounding structures virtually defines many of the symptoms to be
recognized as related to the malignancy.
One of the characteristics that define many malignancies capable of metastasizing is their ability to shed surface membrane proteins into the blood stream where they can be detected as so-called tumor markers. The primary clinical importance associated with defining these tumor markers, many representing carbohydrate antigens, has been to be able to monitor the status of the patient in terms of response or lack of to therapy. As for the Ca 19.9 [4,10] carbohydrate marker representing a carbohydrate antigen at the tumor surface, this molecule is altered under a number of different situations including cancer and inflammation of the organ. As such, without a clinical histologic diagnosis, its presence can’t guarantee a malignant status. Once the diagnosis of the disease is established, an elevation in such markers does signify failure to respond to therapy. The tumor markers defined by our group at Precision Biologics are protein in origin representing one of several oncofetal proteins and are not carbohydrate based. They are sensitive as well as specific to the tumor and highly accurate in defining the existence of a malignancy.
What makes the tumor markers developed by Precision Biologics significant is that they represent the immunogenic protein of the cell that characterizes the tumor system in which they are expressed [11,12]. For each tumor system it seems that the characterizing protein representing tumor type is specific for that tumor without cross reactivity to the surrounding normal tissue. This protein is immunogenic and in most instances oncofetal in origin. The failure to have noted their presence as a primary signal for turning on the host immune system was first alluded to by Prehn [13]. He suggested their existence being an important factor in the behavior of the tumor, but was unable to clinically define them in patients. Rather he believed that all malignancies were characterized by such oncofetal proteins but that they were present at such low levels as to go unrecognized by the host’s immune system. Experiments that he performed confirmed that such a protein did exist but required pooling to reach a threshold level for effectiveness. Once attained, it could, on a theoretical basis, be employed as a vaccine to contain or eliminate the tumor. Such experiments with human tumor specimens further confirmed Prehns hypothesis that all tumors contained such immunotherapeutic proteins, but at levels far below the threshold level needed for immune recognition.
Discussion
In the 1970’s and 80’s with approval from the FDA, Hollinshead at George Washington Univ. was able to validate Prehns proposals by preparing pooled allogeneic tumor membrane preparations from an array of human neoplasms collected at the time of surgery. These pooled proteins were separated and first identified by their M.W. using Sephadex gel followed by discontinuous polyacrylamide gel electrophoresis. The latter approach was used to narrow down the true immunogen present in the crude preparation [14].
To be able to confirm the specific activity of a protein group or lack of such activity to the antigen being evaluated, patients were subjected to skin testing for delayed hypersensitivity reactions. (DHR) among those with the corresponding tumor of interest (colon cancer), a separate group of patients with malignancies other than that of the primary as well as normal volunteers.
When the proper functional protein group was defined, a vaccine preparation was developed for clinical testing. Doses of 300 ugm. Were employed singly and in combination to define the therapeutic level needed. The results, following administration of the vaccine at the 900 ugm. Level revealed a dramatic improvement in survival of the treated group over the 5-7 years of the studies that were performed. An analysis of the improved clinical results suggested that the major antitumor response was a result of the production of significant levels of IgG1 that targeted the lesion, The mechanism for tumor destruction was determined to be antibody dependent cell cytotoxicity (ADCC) although some degree of apoptosis that was noted resulted from the phenomenon produced via annex in V binding. This suggested that cytotoxicity resulted when the tumor membrane became porous allowing phospholipid serine to migrate from the inner cell surface membrane to the outer cell membrane possibly as a result of TRAIL ligation (Figure 3).
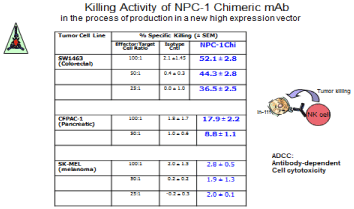
Figure 3: Demonstrates the ADCC killing capacity of NPC-1 in CF PAC-1
pancreatic cancer cell lines.
The ADCC mechanism was clearly shown to represent the effect of NK cell activity though there is evidence that while NO toxicity and perforin can induce the cell destruction seen, that nitric oxide synthase, also arising from the NK cell can also play a role possibly inducing a state of dormancy in many of the patients..
When using the monoclonals to achieve an antitumor response it is necessary to employ the chimeric or human format of the mAb. As the mAb (IgG1) circulates following its IV administration, its Fc has the receptor for natural killer cells which are then delivered by the tumor specific mAb to the malignant cell surface. The ADCC study is performed with the effector cells that are delivered containing a defined number of NK cells. In order to properly quantitate them, the cells are reported as an E: T ratio or the number of effector cells to the tumor cells in culture.
Mesothelin, first isolated at Johns Hopkins was also shown to be one of the markers of some importance in defining the development of the pancreatic cancer lesion but over the last decade has slowly fallen out of favor when used in an attempt at diagnosing pancreatic cancer [6]. In general, the clinical serum markers presently available for clinical use are all carbohydrate in origin, showing up in many conditions unrelated to cancer. Their use as such has been relegated to monitoring the clinical course of a known malignancy in terms of response or lack of, to a therapeutic approach. In terms of pancreatic cancer, the approach employed for following such patients subsequent to having established a clinical diagnosis, is the use of an array of monoclonal antibodies to detect circulating tumor markers. The more commonly utilized antibodies (mAb’s) employed in most studies are CA.19.9 as well as CA-50, and CA-195. The antigens that we have defined in pancreas cancer have also been shown to shed into the serum as well as cystic fluid associated with pancreatic cysts. They can be detected with a high degree of accuracy when employing the related monoclonals in a serum ELISA.
Our group has been interested in defining more effective targets that were found to be specific to pancreas cancer without showing cross reactivity to normal pancreatic tissue [15,16]. We were able isolate, define and characterize several antigens, that is tumor membrane proteins, that proved to be immunogenic and showed no evidence of cross reactivity to normal tissues. These antigens which represent oncofetal proteins were first defined by our group in colorectal cancer but shown to have a strong presence in pancreatic cancer. The major antigen that we are presently working with in preparing for clinical vaccine trials is a post translational modification of MUC5ac needed by the fetus for production of mucin [17]. At birth the gene is remethylated to stop functioning. Failure to do so can lead to the condition- cystic fibrosis. Studies at present are utilizing monoclonals that define and attack cells expressing the MUC5ac mutated molecule acting as the oncofetal protein for a large number of metastatic pancreatic cancer patients.
In pancreatic cancer, as the molecular process associated with transformation to malignancy evolves, those intraepithelial cells involved in the process of transformation begin to express tumor associated antigens (TAA’s). The tumor associated proteins that we have been working with were shown as previously mentioned to be oncofetal in origin. There were 3 primary target proteins found which were present singly or in various combinations. They represent mutated or post-translational modifications of MUC5ac, CEAcam 5-6 and an A33-exosome they appear early in the genotypic transformation of the normal pancreatic acinar cells to their malignant state. The nature of these oncofetal proteins and the monoclonals derived from them will be covered in more detail. These proteins were among the immunogens first detected in the original Hollinshed Colon Cancer Vaccine preparation which in the process of purification appeared as a single band on discontinuous polyacrylamide electrophoresis. Later it was shown to be a complex of similarly charged molecules when evaluated by HPLC.
By definition, pancreatic cancer is an aggressive lesion that when identified at the time of its initial presentation has a poor prognosis. The overall survival for pancreatic cancer is about 3.5 months taking into account attempts at early diagnosis as well as use of radiation to control the disease. If the patient becomes a candidate for surgical resection due to a favorable clinical evaluation at the time of presentation, within 2 years post op, virtually 90% of such patients will have recurrent tumor.
The primary lesion itself begins within the ductal system of the pancreas as noted above. Those cells involved in the process remains in a relatively dormant state for about 15 or more years which should give the clinician ample time to discover and define such premalignant lesions at a time when cure is relatively high. In patients with a familial history of pancreatic Ca, malignant melanoma and with a clinical history of pancreatic inflammatory disease, trans duodenal ductal brushing with evaluation of the cellular material by immunohistochemistry should offer the opportunity for detection of the premalignant cells slowly transforming within the ductal system. Surgery or introduction of radio labeled material into the ductal system should present the possibility of eradicating such cells and as such minimize their incidence of transformation to full malignancy.
Among the less common lesions of pancreas that one may encounter, those patients with neuroendocrine tumors have a much more favorable prognosis than, for example, those with adenocarcinoma of the pancreas. The natural history of neuroendocrine tumors, islet cell tumors, and carcinoid tumors tends to be very different than that of pancreatic adenocarcinoma [18,19]. For example, the median survival duration from the time of diagnosis for patients with non-functioning metastatic islet cell tumors approaches five years. As an organ situated for the most part in the region of abdomen/retroperitoneum (lesser sac), the pancreas extends across the upper abdomen from the duodenal sweep to the spleen. The organ is divided into 3 structures, the head, body and tail. Tumor arising at each site presents in a different fashion the symptoms for each site. Remain different. They usually are noted when the tumor presents somewhat late in its course of clinical development. In the head region it is painless jaundice, in the body, invasion of coeliac axis causing severe back pain and in the tail, when the splenic vein is invaded, gastric varices develop along the greater curvature of the stomach and gastric bleeding has started.
The surgical procedures employed are most frequently associated with early presentation of lesions developing in the pancreatic head [20]. For the majority of patients with tumors of the head region painless jaundice is most commonly noted. The one exception is when the tumor arises in the uncinate process. Should any evidence of spread beyond the primary site be seen then the lesion is considered inoperable. At times should the superior mesenteric vein be compromised when trying to create the tunnel under the pancreatic neck then RT can be used in an attempt to convert the lesion to operability. Division of the neck not only sets up the end phase of the Whipple procedure where the pancreatic head and duodenum can be removed effectively, but the maneuver exposes the length of superior mesenteric vein as it courses superiorly to become the portal vein. Most of the patients seen do require some form of neoadjuvant chemotherapy early on in their management. The end results for most of the patients are really measured in months of questionable improvement.
Therapeutic Approaches in Managing Pancreatic Cancer
Five types of standard treatment are used
- Surgery
- Radiation therapy
- Chemotherapy
- Chemoradiation therapy
- Targeted therapy
- Biologic therapy
- Niederhuber JE, Brennan MF, Menck HR. The National Cancer Data Base report on pancreatic cancer. Cancer. 1995; 76: 1671-1677.
- Michaud DS. Epidemiology of pancreatic cancer. Minerva Chir. 2004; 59: 99- 111.
- Nagakawa T, Nagamori M, Futakami F, Tsukioka Y, Kayahara M, Ohta T, Ueno K. Results of extensive surgery for pancreatic carcinoma. Cancer. 1996; 77: 640-645.
- Ritts RE, Pitt HA. CA 19-9 in pancreatic cancer. Surg Oncol Clin N Am. 1998; 7: 93-101.
- Ozkan H, Kaya M, Cengiz A. Comparison of tumor marker CA 242 with CA 19-9 and carcinoembryonic antigen (CEA) in pancreatic cancer. Hepatogastroenterology. 2003; 50: 1669-1674.
- Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et.al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression. Clin Cancer Res. 2001; 7: 3862-3868.
- Hruban RH, Takaori M, Kyoichi DS, Adsay NV, Albores-Saavedra J, Biankin AV, et.al, An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004; 28: 977-987.
- Hruban RH, Iacobuzio-Donahue C, Wilentz RE, Goggins M, Kern SE. Molecular pathology of pancreatic cancer. Cancer J. 2001; 7: 251-258.
- Gress TM, Müller-Pillasch F, Geng M, Zimmerhackl F, Zehetner G, Friess H, et al. A pancreatic cancer-specific expression profile. Oncogene. 1996; 13: 1819-1830.
- Arlen M, Arlen P, Wang X, Saric O, Martin DA, Deutsch G, et al. The clinical detection of pancreatic carcinoma: a comparison of the standard biomarkers to that of a newer class of biomarkers used for both diagnosis and therapy. Pancreatic Dis Ther. 2013; S4: 001.
- Arlen M, Arlen P, Crawford J, Coppa G, Saric O, Bandovic J, et al. The nature and function of immunogenic tumor proteins that characterize pancreatic and colorectal cancer: a review. J Clin Cell Immunol. 2015; 6: 367.
- Bartal K, Tsang O, Saric M, Wooding R, Barnadjian C, Goldstein Y, et al. Monoclonal Antibody defining an antigen present withinpurified tumor membrane vaccine. Proc. Am. Assoc. Cancer Research.1990; 1539.
- Prehn RT, Main JM. Immunity to methylcholanthrene-induced sarcomas. J Natl Cancer Inst. 1957; 18: 769-778.
- Arlen M, Hollinshead AC, Tsang KY. Identification and characterization of a colon TAA Annals N.Y. Academy of Science. 1993.
- Arlen M, Tsang K. The nature of the monoclonal antibody derived from immunogenic membrane antigen of human colon carcinoma origin. J. Tumor Marker Oncology. 1990. 5: 313-319.
- Adams GP, Weiner LM. Monoclonal antibody therapy of cancer. Nat Biotechnol. 2005; 23: 1147-1157.
- Patel SP, Bristol A, Saric O, Wang XP, Dubeykovskiy A, Arlen PM, et al. Anti-tumor activity of a novel monoclonal antibody NPC-1C optimized for recognition of tumor antigen MUC5ac variant in preclinical models. Cancer Immunol Immunother. 2013; 62: 1011-1019.
- Lynch HT, Smyrk T, Kern SE, Hruban RH, Lightdale CJ, Lemon SJ, et al. Familial pancreatic cancer: a review. Semin Oncol. 1996; 23: 251-275.
- Lowenfels AB, Maisonneuve P. Hereditary pancreatitis and the risk of pancreatic cancer. Gastroenterology. 1995; 109: 247-251.
- Sarr MG, Cameron JL. Surgical management of unresectable carcinoma of the pancreas. Surgery. 1982; 91: 123-133.
- Ahlgren JD. Chemotherapy for pancreatic carcinoma. Cancer. 1996; 78: 654- 663.
- Arlen M, Arlen P. Optimizing the immune system to achieve control of the metastatic malignant lesion. J Cancer. 2013; 4: 427-432.
- Morse MA, Hall JR, Plate JM. Countering tumor-induced immunosuppression during immunotherapy for pancreatic cancer. Expert Opin Biol Ther. 2009; 9: 331-339.
Most protocols are designed to cover those having failed standard treatment which includes FOLFIERI followed by Gemzar and Abraxane. Some of the newer treatments are being tested in clinical trials for the failure groups only. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment.
Use of chemotherapy
Chemotherapy is used in an attempt to prevent growth of cancer cells, either by killing the cells or by inhibition of their dividing mechanisms [21]. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells at all potential metastatic sites (systemic chemotherapy). The reservoir for many of the circulating cells has been proven to be bone marrow. Should there be any locally traumatic site releasing transforming growth factor Beta or other tumor cell stimulant, then cells can leave the marrow to migrate to the site of maximum inflammation.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells without harming normal cells. Tyrosine kinase inhibitors (TKIs) are targeted therapy drugs that block signals needed for tumors to grow. Erlotinib is a type of TKI used to treat pancreatic cancer.
Biologic therapy is a form of treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This type of cancer treatment is also called biotherapy or immunotherapy. In order to be effective the approach must target a molecule expressed in the tumor that is immunogenic and specifically characterizes that malignancy and not normal tissue from which the tumor has been derived (Figure 4).
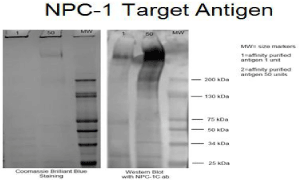
Figure 4: Gel fractionation defining the antigen as one with an MW of approx
600kd.
Patients can enter most clinical trials before, during, or after starting their initial cancer treatment. At Precision Biologics we are working with monoclonal antibodies that target and destroy the cancer cell via ADCC [22]. These antibodies were originally developed to target the Holllinshead TAA preparation consisting of pooled allogeneic membrane protein. The initial intent for producing these monoclonals was to use them in the process of purifying antigen for eventual sequencing with the goal of developing a recombinant vaccine product. The material derived from pooled human tumor specimens was fractioned on a Sephadex column. And then further purified by isoelectric focusing to obtain a single band of approximately 600 kd. A Western blot of the antigenic material indicates that the MUC5ac (NPC-1) antigen component roughly measured 600kd in size.
The single band when further characterized by HPLC (Figure 5) was found to represent several proteins migrating to the area where several proteins of similar charge were noted. Three specific oncofetal proteins were defined and found to be present in both colon and pancreatic carcinoma singly and in different combinations. No cross reactivity to normal tissue was noted. This was important since in an attempt to use the immunogens for immunotherapy in the form of a vaccine in the original vaccine trial during performed during the 1980’s, oil based adjuvant was needed to prevent rapid loss of antigen at the immunization site.
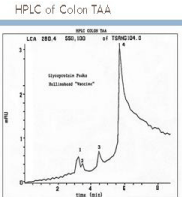
Figure 5: HPLC of single band protein indicating several immunogenic bands.
When the original colon and pancreatic cancer vaccines were first delivered to patients it was noted that the antigen dissapated from the inoculation site within hrs of administration. It was decided that a solubilized antigen preparation was disseminated too quickly for proper processing and needed the addition of an oil based adjuvant to contain the vaccine at the site of inoculation. Of the adjuvants examined, complete Freund’s adjuvant was felt to offer the best possibility for helping to stimulate an effective immune response. Since normal tissue homogenized with Freund’s adjuvant will destroy the normal organs from which the tissue was derived, FDA felt that if the pancreas TAA supposedly specific to the tumor also had cross reactivity to normal tissue, severe immune complications could occur. When finally approved, no incidence of inflammation of normal tissue was seen. There were no cases of induced colitis with diarrhea, pneumonia or pancreatitis when patients received the corresponding vaccine containing complete Freund’s adjuvant.
The initial clinical trials were initiated with the Hollinshead vaccines in the mid 1970’s and 80’s. While relatively pure and free of bacterial and viral contamination, they were produced in the research lab at George Washington University, without requirement of GMP production. Based on favorable clinical results after 7 years of use in patients, FDA was approached to allow scale up of the vaccine for approval for commercialization The application for using a pooled allogeneic preparation was rejected because of the recent recognition in the late 1980’s of HIV, HPV, Hepatitis C and other viral pathogens that could be incorporated into a vaccine produced from pooled allogeneic operative tumor specimens. Rather, it was requested that we develop a recombinant vaccines for patients use.
It was apparent following the FDA meeting that we needed to further purify the partially purified vaccine material to allow sequencing to occur. Monoclonal antibodies as such, were produced (Figure 6) at which time 3 discrete antibody groups were noted each demonstrating activity against the Hollinshead antigen used in the hybridoma production. The mAbs corresponded to what we had noted on HPLC of the antigenic vaccines being employed each peak could be matched with a corresponding monoclonal antibody that we had produced.
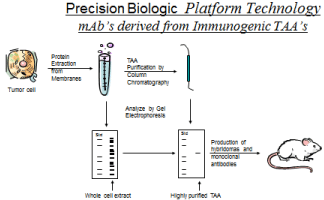
Figure 6: Development of Immunogenic proteins used for mAb production
capable of diagnosis and therapy with no cross reactivity to normal tissue.
Almost simultaneously we analyzed the results of the initial vaccine trials which revealed between an 80 and 90% freedom of disease at 7 years. The failure of the 10-20% of patients to remain free of disease appeared to be related, not to an absence of an effective cytotoxic T cell response but rather to the failure of those patients to produce an effective IgG1. Since we had already produced the needed mAbs for evaluation of the tumor immunogen, (Figure 7) animal studies were performed with transplanted human colon and pancreas cancer in nude mice. In those animals reaching near end stage disease, 400 ugm of monoclonal antibody resulted in an almost total elimination of tumor by 2wks post peritoneal infusion of the monoclonal antibody [23].

Figure 7: Effect on pancreatic tumor transplants using colon/pancreas mAb
neo 102.
After extensive studies related to one of our monoclonals NPC- 1, as a therapeutic agent targeting MUC5ac expressed in metastatic pancreatic cancer, an IND was submitted to the FDA for treatment of patients with metastatic pancreas cancer having failed all forms of chemotherapy. In particular those patients who we planned to enter into the clinical study would represent those showing progression of disease and where chemo drugs such as Gemzar and Abraxane combinations had failed. Such patients had no more than several weeks of anticipated survival. When Abraxane was added to those patients having failed standard Gemcitabine, this latter additional form of therapy added about 7 wks toward prolongation of their survival. Those chosen to receive therapeutic antibody treatment were chosen based on high levels of tumor antigen targeted by the antibody. The trial was designed to evaluate how this form of immunotherapy could enhance or prolong survival among this group of patients with the situation such that we had already produced monoclonal antibodies against the target protein for purification purposes we were able to initiate further animal studies with the mAbs (Figure 8 and 9) to confirm their antitumor activity. In vivo models were designed using pancreatic tumor transplants in animals to define the efficacy of an IgG response.
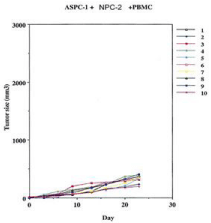
Figure 8: Addition of non specific IgG with effector cells on tumor growth.
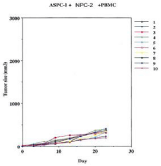
Figure 9: The effect of specific mAb targeting Muc5ac given with effector
cells. A marked reduction in tumor growth seen.
We were able to convince FDA at this point that we should consider use of our monoclonals as possible therapeutic agents for the treatment of advanced pancreatic cancer and that an IND would be filed. Phase I trials were begun with a GMP product. By choosing patients having failed all therapeutic modalities it was essential to obtain a rapid antitumor response which could only be accomplished with IV monoclonals that target the tumor immunogen rather than use of a vaccine which takes about 4 months to achieve proper serum titers of the antibody. For patients undergoing a Whipple procedure, post op vaccine therapy would of course be more effective and is presently in the planning stages.
The response to IV monoclonal therapy appeared far better than anticipated using terminal patients with a naked antibody alone. 40 patients have been entered into the ongoing trial and the data slowly collected. There is evidence of an effective response where survival to date has improve significantly by at least 150% or more. The data is being analyzed as more patients are being entered into phase II. Essentially no toxicity has been noted. Discussions are ongoing to begin a randomized trial with chemo vs. immunochemo in patients with earlier signs of recurrent disease such that if patients have failed FOLFIERI then they will be randomized to Gemzar Abraxane vs. Chemo Immunotherapy. The purpose of the chemo in the latter arm is to minimize the shedding of inhibitory material which can then impede the effect of ADCC.
Considering that the sequence of one of our tumor associated antigens can be provided for constructing a proper vaccine to be employed in the adjuvant setting, this type of molecule would probably be non functional since the structure would be produced in a linear format. Such molecules require proper folding at the endoplasmic reticulum and as such not achievable. We have however, used phage display and have found that our antibody binds to a 12 mer peptide. This type of peptide vaccine has been produced, tested and found functional. In the near future we will be delivering post op vaccine following Whipple pancreatectomy to reduce if not eliminates the high recurrence seen following this type of surgery.
This type of vaccine may be also being developed as a variation in the MAP peptide format.
When the monoclonal targets the malignant disease process it essentially defines the immunogenic protein expressed exclusively within the tumor cell and does not effect adjacent normal tissue compared with those mAbs targeting growth factors the incidence of normal tissue toxicity if negligible or nonexistent (Figure 10).
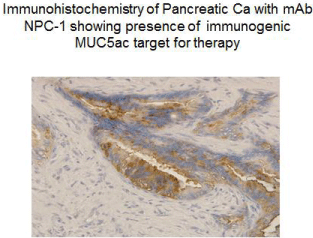
Figure 10: The antigen expressed in the primary tumor does not mutate
so that the same antigen is present in the metastatic lesion to define that
monoclonal that can be used for treatment.
With the clinical data that we have initially obtained showing a significant improvement in survival of metastatic pancreatic cancer having failed all forms of therapy including Gemzar and Abraxane, our products should eventually demonstrate further improvement in survival as we enter Phase III. Overall enhancement will eventually be obtained using IL-15 to increase the ADCC effect and the eventual addition of a radiolabelled alpha emitter in the later stages of the clinical protocol.
References