
Case Report
Austin J Gastroenterol. 2016; 3(3): 1069.
Endoscopic Submucosal Dissection of a Gastric Cardia Cancer Demonstrating Distinctive Progression from a Small Hyperplastic Polyp
Yoshida K¹, Omori K¹, Kamei T², Shimamoto D³ and Kan M¹*
¹Department of Gastroenterology and Hepatology, Sato Daiichi Hospital, Japan
²Medical Director of Pathology & Cytology Center BML group PCL, Japan
³Department of Radiology, Sato Daiichi Hospital, Japan
*Corresponding author: Masahiro Kan, Department of Gastroenterology and Hepatology, Sato Daiichi Hospital, 77-1 Hokyoji, Usa-Shi, Oita 879-0454, Japan
Received: August 10, 2016; Accepted: October 10, 2016; Published: October 13, 2016
Abstract
We report a rare case of endoscopic observations of the distinctive progression of a small hyperplastic polyp (HP) with low grade dysplasia to early gastric cardia cancer in a background of atrophic pangastritis that led to an uninhabitable state for Helicobacter pylori, over the course of thirty months with no obvious superficial morphological changes, and it was removed en bloc by endoscopic submucosal dissection using the insulation-tipped diathermic knife-2. The resected lesion was histologically diagnosed as well-differentiated adenocarcinoma mostly with some moderate-differentiated adenocarcinoma extending 400 μm into the submucosal layer without vessel infiltrations, in which hyperplastic components were not detected. There were features of both the background atrophic pangastritis that led to an uninhabitable state for Helicobacter pylori and cardia location, which may be a part of the various processes underlying the distinctive progression. One growth process of gastric cardia cancers was clarified in our case. Therefore, if atypical cells are observed even in small HPs, particularly with features as observed in our case, endoscopic resection should be considered prophylactically regardless of the presence of gross morphological changes, while performing yearly surveillance.
Keywords: Hyperplastic polyp; Gastric cardia cancer; Insulation-tipped diathermic knife-2; Helicobacter pylori; Endoscopic submucosal dissection
Case Presentation
An 80-year-old woman with hypertension was being followed up at our hospital. There were no abnormalities revealed on physical and routine laboratory examinations; the latter included blood biochemistry assessment to detect serum tumor markers, such as carcinoembryonic antigen and carbohydrate antigen 19- 9. Surveillance upper gastrointestinal endoscopy was performed. Observations using conventional endoscopy revealed a reddish polyp with a shallow depression at the top of the lesion having 5 mm diameter located beneath the gastroesophageal junction (GEJ) in a background of atrophic pangastritis (Figure 1), when Barrett’s esophagus was not observed. The lesion did not present signs of carcinoma on endoscopic observations including unmagnified narrow-band imaging (NBI) (Figure 1). Biopsy samples were obtained from four parts including the shallow depression, and the lesion was eventually diagnosed as a hyperplastic polyp (HP) with low grade dysplasia (Figure 2). Considering these features of the lesion, we thought that the risk of cancer was low, and chose to perform yearly surveillance. Although there was no history of eradication of Helicobacter pylori (H. pylori), current H. pylori infection was not detected on culturing; by rapid urease, urea breath, or on microscopic examination. Laboratory findings were serum pepsinogen I of 10.1 ng/ mL (normal value: =70 ng/mL), pepsinogen I/II ratio of 2.1(normal ratio: =3.0), a negative H. pylori antibody, a negative anti-intrinsic factor antibody, and a negative anti-gastric parietal cell antibody. One year later, the lesion was biopsied and sequentially diagnosed as a hyperplastic polyp with low grade dysplasia. Thirty months later, the lesion was believed to show a mild fullness, while maintaining its small size (Figure 1). The lesion was biopsied and diagnosed to be a well-differentiated adenocarcinoma. The HP transformed into a small well-differentiated adenocarcinoma, however, there were almost no appreciable superficial morphological changes associated with this progression during the thirty months (Figure 1). Endoscopic ultrasonography was performed to evaluate the invasion depth, and the cancer was classified to be an intramucosal carcinoma. Endoscopic submucosal dissection (ESD) was believed to be feasible according to the Japanese Gastric Cancer Treatment Guideline [1]. The cancer was removed en bloc by ESD using the insulation-tipped diathermic (IT) knife-2 (KD-611 L; Olympus Medical Systems, Tokyo, Japan) and the transparent hood (F-030; TOP Co., Ltd., Tokyo, Japan) (Figure 3). The resected lesion was histologically diagnosed as well-differentiated adenocarcinoma mostly with some moderate-differentiated adenocarcinoma, comprising intramucosal carcinoma mostly with a submucosal invasion depth of 400 μm in a small range, in which hyperplastic components and vessel infiltrations were not detected (Figure 4). The resection was considered as curative according to the Japanese Gastric Cancer Treatment Guideline [1]. Neither recurrence nor metastasis of gastric cardia cancer has been detected at present, i.e., two years later.
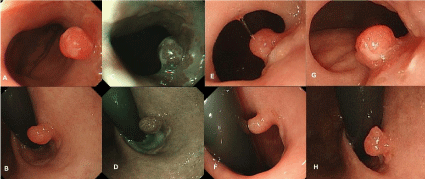
Figure 1: (A) (B) Conventional view of a small, reddish hyperplastic polyp with a shallow depression at the top of the lesion approximately 5mm in diameter
located beneath the gastroesophageal junction at the first time. (C) (D) Endoscopic view of a hyperplastic polyp with narrow band imaging at the first time. (E) (F)
Conventional view of the hyperplastic polyp after one year. The lesion was observed without considerable morphological changes. (G) (H) Conventional view of a
small gastric cardia cancer arising from the hyperplastic polyp after thirty months. The lesion was found to be a mild fullness without considerable morphological
changes.
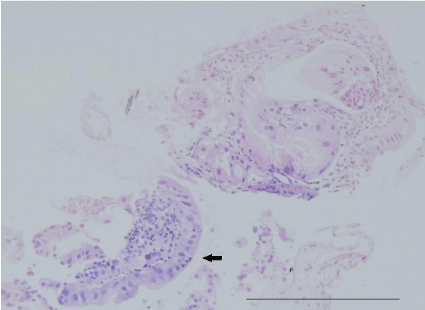
Figure 2: Pathological findings of the first biopsy specimen stained with
hematoxylin-eosin revealed a hyperplastic polyp with low grade dysplasia
(black arrow).Bar =100 μm.
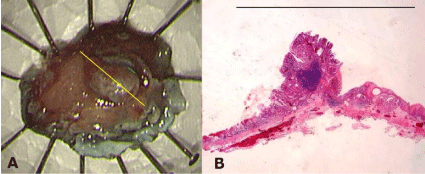
Figure 3: (A) The endoscopic submucosal dissection specimen. (B) A
macroscopic view of the resected specimen showing well-differentiated
adenocarcinoma comprising intramucosal carcinoma (yellow line). Bar = 10
mm.
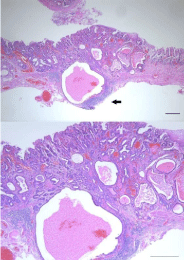
Figure 4: Hematoxylin-eosin staining of the section of the resected specimen
revealed well-differentiated adenocarcinoma mostly with some moderatedifferentiated
adenocarcinoma that extended into the submucosal layer to
a depth of 400 μm in a small range (black arrow) without vessel infiltrations
(green line in Figure 3). Bar =100 μm.
Discussion
We witnessed the distinctive progression of a small HP with low grade dysplasia into early gastric cardia cancer without appreciable superficial morphological changes, in a background of atrophic pangastritis that led to an uninhabitable state for H. pylori, which could be endoscopically visualized, and the entire lesion was subsequently removed by ESD. Gastric HPs typically occur in the antrum and corpus and also in the GEJ region, albeit less frequently. Chronic inflammatory disorders of the stomach, such as H. pylori-associated gastritis, pernicious anemia, and reactive or chemical gastritis, have been reported to be strongly associated with HPs [2,3]. In our case, the patient was not pernicious anemia, and auto-antibodies suggestive of autoimmune gastritis were negative. H. pylori-associated gastritis is generally considered to promote proliferation in the mucosal epithelium, leading to the development of HPs [2]. However, HPs in the cardia are reported to be correlated with chronic esophageal reflux, independent of the status of H. pylori infection [4]. Many epidemiological studies have demonstrated a close correlation between H. Pylori infection and distal gastric cancers, whereas it appears unlikely that H. Pylori infection is a primary factor contributing to the rising incidence of gastric cardia cancers [5]. Gastric cardia cancers have been reported to closely correlate with Barrett’s esophagus and esophageal reflux in the West [5]. With presumably distinct initiating and promotional factors, the characteristics of both HPs and gastric cancers in the cardia may differ from those in other gastric sites. However, atrophic gastritis is reported to increase the risk of gastric cardia adenocarcinoma as well as non-cardia gastric adenocarcinoma [6]. Our case was considered to present with the condition of atrophic pangastritis that led to an uninhabitable state for H. Pylori because of the endoscopic finding (an extended atrophic mucosa), and laboratory findings (a negative current H. pylori infection, low serum pepsinogen I, low pepsinogen I/II ratio and negative serum H. pylori antibody), where it has been reported to be an extremely high risk factor for the development of gastric cancer [7]. Although HPs were traditionally considered to lack malignant potential, a low risk of carcinomatous conversion is now recognized [8]. Gastric cancer has been reported to coexist within 1%-3% of HPs with a diameter of =10 mm [9]. However, there are few reports of a malignant transformation of a small size HP as observed in our case. Endoscopic features such as large granular structures or a depression on the surface of the polyp have been reported to be associated with the malignant transformation of HPs [9]. Because it is known that non-representative biopsies occur frequently, four biopsies at least are recommended to improve the histologic accuracy [10]. In our case, biopsy samples were obtained from four parts including the shallow depression, and the lesion was eventually diagnosed as an HP with low grade dysplasia, considering the endoscopic observations including unmagnified NBI. Considering the presence of low grade dysplasia, the growth process of gastric cancer in our case may be explained by the hyperplasiaadenoma- adenocarcinoma sequence, which had been reported to be the mechanism of malignant transformation of gastric HPs [8]. In the present case, the cancer was considered to have high malignant potential due to its rapid extension into the submucosal layer with no appreciable superficial morphologic changes including its small size. This case suggests that morphological changes alone are not absolutely critical when deciding on the best approach for managing HPs. Although magnified endoscopy is not widely used, magnified endoscopy including NBI may be useful to detect cancer earlier [9]. As observed in our case, the features of both the background atrophic pangastritis with an extremely high risk factor for the development of gastric cancer and cardia location with distinctive initiating and promotional factors for gastric cancers may be a part of the various processes underlying the distinctive progression. The incidence of gastric cancers in the upper third of the stomach has been reported to be increasing [11]. Interestingly, one growth process of gastric cardia cancers, in which the cancer arose from a small HP and hyperplastic components in the cancer disappeared sequentially with no appreciable superficial morphologic changes within thirty months, was clarified in our case. ESD for early gastric cancers has been widely accepted as a standard treatment, while improving efficiency and safety of ESD. Selection of the proper knife, such as the needle, IT knife-2, hook, flex, and flush, influences the quality of the ESD procedure and overall outcome. In our case, use of the IT knife-2 contributed to the safe and effective ESD for early gastric cardia cancer.
Conclusion
There is controversy regarding clinical management of HPs; some authors recommended performing polypectomy for all small HPs and periodic biopsy of large HPs, while others recommended only large HPs should be removed, as cancer usually occurs in bigger HPs [12]. Considering the present case, If atypical cells are observed even in small HPs, particularly with features as observed in our case, endoscopic resection should be considered prophylactically regardless of the presence of gross morphological changes according to guidelines that have recommended that all gastric polyps with dysplastic foci should be completely removed [12,13], while performing yearly surveillance.
References
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011; 14: 113-123.
- Jain R, Chetty R. Gastric hyperplastic polyps: a review. Dig Dis Sci. 2009; 54: 1839-1846.
- Park DY, Lauwers GY. Gastric polyps: classification and management. Arch Pathol Lab Med. 2008; 132: 633-640.
- Abraham SC, Singh VK, Yardley JH, Wu TT. Hyperplastic polyps of the esophagus and esophagogastric junction: histologic and clinicopathologic findings. Am J Surg Pathol. 2001; 25: 1180-1187.
- Okabayashi T, Gotoda T, Kondo H, Inui T, Ono H, Saito D, et al. Early carcinoma of the gastric cardia in Japan: is it different from that in the West?. Cancer. 2000; 89: 2555-2559.
- Islami F, Sheikhattari P, Ren JS, Kamangar F. Gastric atrophy and risk of oesophageal cancer and gastric cardia adenocarcinoma--a systematic review and meta-analysis. Ann Oncol. 2011; 22: 754-760.
- Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, et al. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005; 54: 764-768.
- Imura J, Hayashi S, Ichikawa K, Miwa S, Nakajima T, Nomoto K, et al. Malignant transformation of hyperplastic gastric polyps: An immunohistochemical and pathological study of the changes of neoplastic phenotype. Oncol Lett. 2014; 7: 1459-1463.
- Ishido K, Tanabe S, Hayashi S, Higuchi K, Katada C, Naruke A, et al. A case of early gastric cancer coexisting with a hyperplastic polyp. Kitasato Med J. 2013; 43: 82-85.
- Sung JK. Diagnosis and management of gastric dysplasia. Korean J Intern Med. 2016; 31: 201-209.
- Liu Y, Kaneko S, Sobue T. Trends in reported incidences of gastric cancer by tumour location, from 1975 to 1989 in Japan. Int J Epidemiol. 2004; 33: 808-815.
- Goddard AF, Badreldin R, Pritchard DM, Walker MM, Warren B. British Society of Gastroenterology. The management of gastric polyps. Gut. 2010; 59: 1270-1276.
- Evans JA, Chandrasekhara V, Chathadi KV, Decker GA, Early DS, Fisher DA, et al. The role of endoscopy in the management of premalignant and malignant conditions of the stomach. Gastrointest Endosc. 2015; 82: 1-8.