
Research Article
Austin J Gastroenterol. 2017; 4(4): 1092.
Investigations of Antioxidant and Hepatomodulatory Potentials of Tea (Camellia sinensis) in Black, Green and Purple Tea Fortified Alcoholic Beverages (6% V/V) Using a Mouse Model
Ochanda SO1,4*, Rashid K2, Ngotho M3, Faraj AK4, Wanyoko JK1, Onyango CA5, Maranga DN6 and Wachira FN7
1Department of Tea and Health, Kenya Agricultural and Livestock Research Organization-Tea Research Institute of Kenya, Kenya
2University of Cologne, Albertus-Magnus-Platz, Germany
3Mount Kenya University, Kenya
4Department of Dairy and Food Science and Technology, Kenya
5Taita Taveta University, Kenya
6Department of Animal Sciences, Institute of Primate Research, Kenya
7South Eastern Kenya University, Kenya
*Corresponding author: Ochanda SO, Kenya Agricultural and Livestock Research Organization -Tea Research Institute of Kenya, Kenya
Received: July 28, 2017; Accepted: November 07, 2017; Published: December 22, 2017
Abstract
This study was carried out to evaluate the effects of tea (Camellia sinensis) fortified alcoholic beverages on antioxidant status and liver dysfunction biomarkers in mice. Plain and tea fortified alcoholic beverages at 6% (v/v) alcohol content were administered at a dosage of 1mL per mouse every second day for 4 weeks using a gavage needle after which the animals were euthanized. Albumin, total protein, alkaline phosphatase (ALP) and Glutathione (GSH) levels in serum and liver homogenates were assayed. Consumption of plain alcohols without fortification resulted in a significant decrease (P<0.05) in serum GSH and liver albumin as well as a significant (P<0.05) increase in liver marker enzyme alkaline phosphatase when compared to animals supplemented with tea fortified alcohols or water only. Fortification of alcoholic beverages with tea showed significant protection with lowered liver ALP levels and replenishment of antioxidant status. Moreover, tea fortified alcohols induced a significant (P<0.05) increase in liver albumin when compared to animals fed on plain alcohols, implying a general improvement in the nutritional status of the experimental mice. The findings prove that tea has potent hepatoprotective effects against alcohol induced toxicity. The mechanism of the protective effects may involve augmentation of endogenous antioxidants. Beneficial effects of tea in this study could probably be important for the development of new alcohol products with less deleterious effects.
Keywords: Camellia sinensi; Antioxidants; Alcoholic beverages
Introduction
Alcoholism is posing as a major health problem around the world. This problem is especially prevalent in the African continent and contributes a significant percentage of hospital admission [1]. Metabolism of alcohol is associated with over production of reactive oxygen species (ROS) and subsequent depletion of endogenous antioxidants resulting in oxidative stress [2]. Indeed, a previous study was able to establish that alcohol causes a significant decrease in the levels of key antioxidant enzymes catalase, glutathione peroxidase, and glutathione reductase and superoxide dismutase in rats [3]. Chaturvedi and others corroborated these findings and reported decreased Vitamin C and glutathione levels and a significant increase in the levels of plasma thiobarbituric acid reactive substances in rats fed on alcohol [1]. Consequently as a result of alcohol induced oxidative stress, biological systems are adversely altered with concomitant malfunction of a host of cells and tissues [4]. In this view, therapies aimed against oxidants could be useful in the management of alcohol toxicity.
Nowadays, many therapeutic studies are devoted to phytochemicals due to increased incidences of adverse drug reactions and economic burden on modern systems of medicine [5]. Moreover, a host of plants are rich sources of natural antioxidants with potent radical quenching abilities both in-vivo and in-vitro [6]. Tea (Camellia sinensis) is a perennial tree native to south China and exceptionally rich in antioxidants [7]. Its antioxidant activity is mainly contributed by the high quantities of polyphenols such as flavonol, flavandiols, flavonoids and phenolic acids which constitute more than 30% of the dry weight of the leaves [8]. Tea has for a long time been preferred as a beverage because of its unique flavor and colour. However, the recent research on tea has provided experimental and epidemiological evidence to demonstrate that Camellia sinensis has the ability to boost the body’s antioxidant capacity, making tea a popular health drink [9-12]. Moreover, several studies have appraised the hepatoprotective activity of tea against a number of hepatotoxic chemical agents such as Sodium oxalate [13], Tamoxifen citrate (TAM) [14], Carbon tetrachloride [7], anti-tuberculosis drug Isoniazid-Rifampicin [15], Leflunomide and a combination of Cyromazine and Chlorpyrifos [16]. On the other hand tea which is a natural product has been associated with health benefits because of its high content of phenolic compounds. The health benefits can be transferred to products in which tea has been incorporated. Based on this knowledge, an investigation was made on the hepatoprotective properties of tea against alcohol induced toxicity. Alcoholic beverages fortified with tea were developed and effects of their ingestion tested using mice.
Materials and Methods
Tea samples used in the fortification of the alcoholic beverages
Three different tea types namely aerated black, non-aerated green and non-aerated purple teas were processed and used in the production of the alcoholic beverages used in the research. Raw materials used in the manufacture of the various teas were obtained from the Tea Research Institute (TRI), Timbilil Estate in Kericho (latitude 0°22S, longitude 35°21 E, altitude 2180 m a.m.s.l.). Nonaerated green and aerated black tea was processed from the tea variety TRFK 6/8, while the purple leaf colored variety TRFK 306 was used to process non-aerated purple tea. Freshly harvested young tender shoots comprising of two leaves and a bud were used to process the teas through aeration [17] and non-aeration [18] methods.
Materials for alcoholic beverage production
The ingredients included aerated black and un-aerated green (Clone TRFK 6/8) and non-aerated purple (Clone TRFK 306) Kenyan tea cultivars, milled white sugar, citric acid, raisins, yeast and portable water.
Development and storage of alcoholic beverages
Development of alcoholic beverages was carried out aseptically at the food processing and value addition laboratory of TRI in Kericho, Kenya. Milled white sugar (340 g), raisins (56 g), citric acid (0.5 g), water (1000 mL), yeast (0.8 g) and tea (4 g, 8 g, and 16 g of black, green and purple teas) were mixed together and left to ferment for 14 days. The end products were aseptically filtered, cooled and stored at 20°C. Each set of tea fortified alcoholic beverage had a control without tea.
Experimental animals
All experimental procedures and protocols involving mice strictly adhered to protocols approved by Institutional Animal Care and Use Committee (IACUC) of the National Museums of Kenya-Institute of Primate Research (NMK-IPR), Karen, Kenya. A permit (number IRC/13/12) was obtained for the animal research, prior to the start of the study. A total of 55, eight weeks old female and male adult Swiss white mice weighing between 26-32 g were obtained from IPR rodent breeding colony and used in all experiments. The animals were housed in groups of 5 (different sexes grouped and housed separately), under conventional animal housing conditions within standard mice cages at a temperature of 21-28°C. They were provided ad libitum access to water and standard mice cubes obtained from Unger Feeds Ltd Kenya. Sterile wood-chippings were provided as bedding material. All mice were treated for internal parasites as a precautionary measure using Ivermectin (Ivermectin®, Anupco, Suffolk and England) injected subcutaneously. Carbon dioxide (CO2) was used to euthanize the animals at the end of the experiment as described by Close, et al. [19].
Experimental design
Prior to the start of the research, the study was blinded by random selection of mice and allocation into 11 groups (Table 1) where each animal served as a replicate in a completely randomized design (CRD). The test products, plain and tea fortified alcoholic (6% v/v) beverages were administered orally at a dosage of 1mL per mouse after every second day using a gavage needle. Administration of the test products was continued for 28 days during which time mice were monitored for changes in body weight (bwt) and packed cell volume (PCV). The experiment was terminated through euthanasia 24 h post the last dosage. Liver samples were obtained and whole blood drawn via cardiac puncture, serum separated and stored at -80°C until required for analysis.
Group
Level of fortification with tea
Treatment
0g
1g
2g
4g
Group 1 (Control mice)
-
-
-
-
Water only
Group 2
ü
-
-
-
Plain alcoholic beverage (6%v/v)
Group 3
-
ü
-
-
Black tea alcoholic beverages (6%v/v)
Group 4
-
-
ü
-
Group 5
-
-
-
ü
Group 6
-
ü
-
-
Green tea alcoholic beverages (6%v/v)
Group 7
-
-
ü
-
Group 8
-
-
-
ü
Group 9
-
ü
-
-
Purple tea alcoholic beverages (6%v/v)
Group 10
-
-
ü
-
Group 11
-
-
-
ü
The levels of fortification represent grams of tea in 250 mLof alcoholic beverage
Table 1: Summary of the 11 treatment groups of mice used in the study.
Packed cell volume (PCV) and Body weight (bwt)
At one week interval, PCV was determined as per the method by Hoff and Rlagt [20]. Body weight of each mouse was determined every 2 days using an electronic analytical balance (Mettler PM2000 balance, Ohio, USA).
Liver and blood sample preparation
Frozen whole livers were homogenized at 4°C (on ice) in a buffer that contained 0.25 M sucrose, 5 mM Hepes-Tris, pH 7.4, 1 mM ethylenediaminetetraacetic acid (EDTA) with protease inhibitor cocktail enzymes to a final concentration of 10% (w/v) using a tissue homogenizer (Stuart homogenizer SHM2/UK, Bibby Scientific Limited, USA). The homogenate was aliquoted and stored at -80°C until required for biochemical analysis. Whole blood drawn in 1 mL falcon tubes was left to stand for 1h at room temperature in an upright position for clotting to occur. The serum obtained after blood clotting was then transferred to 1.5 mL microfuge tubes and centrifuged (Heraeus Labofuge 400R, Hanau, Germany) for 15 min at a speed of 1000 xg. Serum was then aliquoted into 1.5mL microfuge tubes and immediately stored at -80°C until required for biochemical analysis.
Glutathione assay
Glutathione assay was performed as described by Rahman, Kode & Biswas, [21] with slight modifications. A volume of 200 μmoL/L of GSH standard solution was prepared in 0.5% sulphosalicylic acid (SSA) and serial dilutions made using the same solution (0.5% SSA) to final concentrations of 100, 50, 25, 12.5, 6.25, 3.13, 1.56 and 0.78 μmoL/L. Ellman’s reagent (5, 5’-Dithiobis (2-nitrobenzoic acid (DTNB)) was prepared by dissolving in 0.1 M potassium phosphate buffer with 5mM EDTA disodium salt, pH 7.5 (KPE buffer) to a final concentration of 0.6 mg/mL. A volume of 50 μL of liver homogenate or serum was mixed with 50 μL solution containing 5% SSA and 0.25 mM EDTA. This mixture was centrifuged at 8000 xg at 4°C for 10 min and 25 μL of the supernatant loaded on a 96-well microtitre plate in triplicate. Approximately 25 μL of each standard and blank were loaded to the remaining wells. To each well, 100 μL of freshly prepared DTNB was then added and the absorbance measured at 405 nm at intervals of 30sec using a multi-detection microtiter plate reader (DYNEX MRX, Vancouver, USA).
Biochemical analyses
A clinical biochemical analyzer (Humalyzer 2000, Wiesbaden, Germany) was used to analyze serum samples and liver homogenates for the estimation of total proteins, albumin and alkaline phosphatase using commercial reagent kits (Human Diagnostics, Wiesbaden, Germany) as described by Heikal, et al. [16].
Data analysis
Data was analyzed using Prism Graph-pad version 5.0 and P<0.05 considered to be statistically significant. Significance of difference between means for glutathione (GSH), total proteins, albumin and liver alkaline phosphatase (ALP) levels were determined by one way ANOVA and Tukey post hoc test was performed to evaluate differences among group means. Graphs were plotted to show the trends of the various response variables. The data were expressed as the mean ± SEM (standard error of the mean).
Results
Packed cell volume (PCV) and body weight (bwt)
Packed cell volume and body weight parameters assayed prior to the start of the experiment were used as base line data. Mice supplemented with tea fortified alcohols recorded a decline in PCV, the degree of drop being dependent on the concentration of tea used in fortification. Oral administration of alcohols fortified with black tea at a concentration of 1, 2 and 4 g/250 mL led to a significant decrease (P<0.05) in PCV levels, reaching the minimum within 17 days post start of administration (Figure 1A). The greatest decrease in PCV was recorded in mice supplemented with black tea alcohols (4 g/250 mL), registering a percentage drop in PCV of 15.49% compared to 4.98% and 9.89% in mice supplemented with black tea alcohols (1g and 2 g/250 mL) respectively. All animals supplemented with green tea alcohols recorded a decline in PCV levels, reaching the minimum within 17 days post start of alcohol administration before rising slightly in the final experimental week. Alcohols fortified with green tea at 2 and 4 g/250 mL decreased PCV levels by 3.95% and 11.45% respectively, while at the lowest tea concentration of 1 g /250 mL, there was a marginal increase in PCV of 2.05% (Figure 1B). Administration of purple tea alcohols significantly reduced PCV values (P<0.05) during the first 10 days of the experimental study when compared to the group supplemented with plain alcohols (positive) or water only (negative) controls. Animals supplemented with purple tea alcohols fortified at 2 g/250 mL registered the highest percentage drop in PCV of 16.88% compared to 7.76%, 12.04% and 3.72% in mice supplemented with purple tea alcohols (1 and 4 g/250 mL) and plain alcohols respectively (Figure 1C). Packed cell volume levels of mice supplemented with water only remained relatively constant with minor fluctuations throughout the experiment.
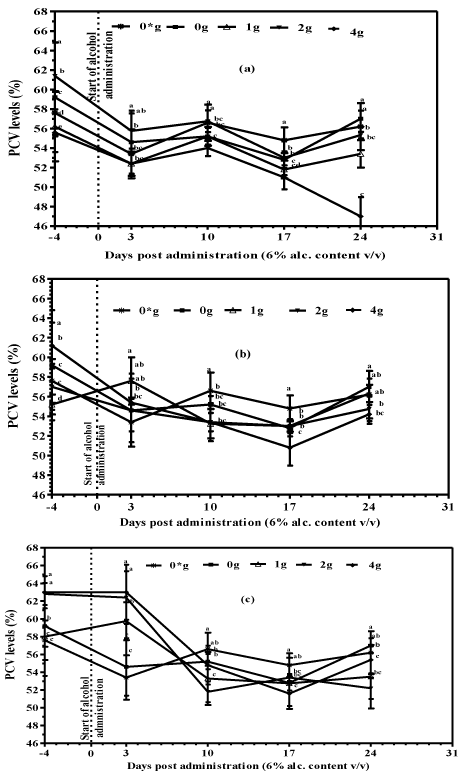
Figure 1: Packed Cell Volume of mice supplemented with tea fortified
alcoholic beverages 6% (v/v) alcohol containing (a) Black (b) Green and (c)
Purple tea as base ingredient at concentrations of 0, 1, 2 and 4 g/250mL (m/v)
and water only (0*) for the negative controls.
Mice supplemented with purple tea alcohols recorded a significant (P<0.05) decrease in PCV% when compared to mice supplemented with plain alcohols or water only for the controls (Figure 2).
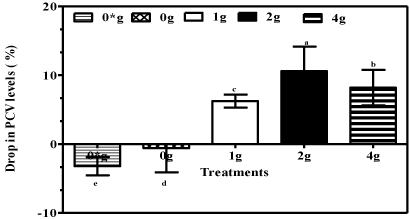
Figure 2: Packed Cell Volume (%) of mice 10 days post start of administration
of 6% (v/v) purple tea fortified alcohols at 0, 1, 2 and 4 g/250mL (m/v) and
water only (0*) for negative controls. Data are means ±standard error of
means (S.E.M) and negative values signify an increase in PCV (%).
There was no significant difference (P>0.05) in mean body weight values between mice supplemented with plain (positive controls) and black (Figure 3A), green (Figure 3B) and purple (Figure 3C) tea fortified alcohols and the controls supplied with water only (negative controls) during the 28 day experimental period. There was however differences in body weights between mice supplemented with different doses of teas which in some cases were significant. The differences could have resulted in part due to the two different sexes (male and female mice) used in the research which could have emanated from the hormonal differences (Figure 3C).
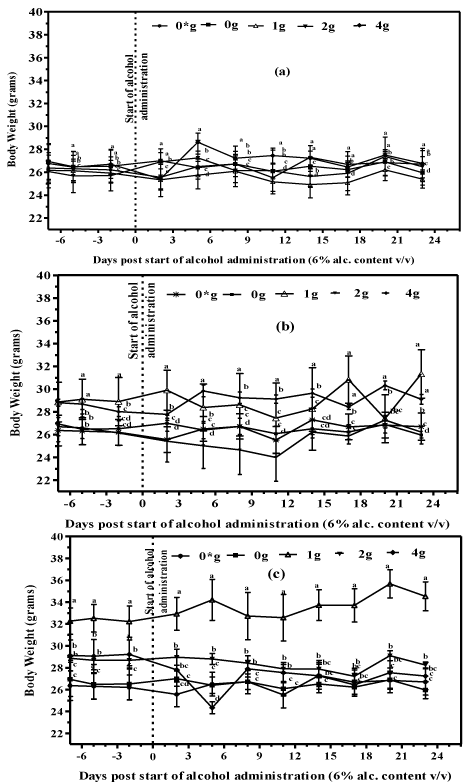
Figure 1: Body weight of mice supplemented with tea fortified alcoholic
beverages 6% (v/v) alcohol containing (a) Black (b) Green and (c) Purple tea
at concentrations of 0, 1, 2 and 4 g/250mL (m/v) and water only (0*) for the
negative controls.
Effects of tea fortified Alcohols 6% (v/v) on total protein in serum
Oral administration of plain alcohols led a marginal reduction in serum proteins, reaching 8.36 g/dL when compared to 9.24 g/dL for mice supplemented with water only (Figure 4). However, fortification of alcohols with green and purple tea had a tendency towards elevated serum protein levels. In fact, experimental animals supplemented with purple tea alcohols (1g /250 mL) recorded significantly higher serum protein levels when compared to mice supplemented with water (P<0.01) or plain alcohols (P<0.001). Mice supplemented with green tea alcohols (1g/250 mL) also recorded significantly (P<0.05) higher serum proteins when compared to animals supplemented with plain alcohols.
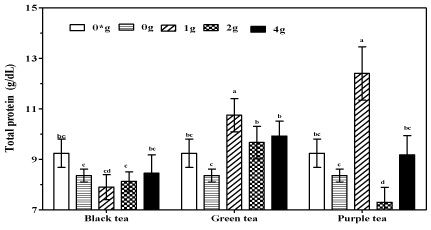
Figure 1: Total protein in serum of mice supplemented tea fortified alcoholic
beverage 6% (v/v) alcohol containing black, green and purple tea at 0, 1, 2
and 4 g/250mL (m/v) and water only (0*) for the negative controls.
Effects of tea fortified alcohols on albumin in serum and liver
Mice supplemented with plain and tea fortified alcohols had significantly (P<0.05) higher serum albumin levels when compared to animals supplemented with water only (Figure 5A). No significant differences (P>0.05) in serum albumin were recorded in animals supplemented with either plain or the various tea fortified alcohols, with an exception in the group supplemented with green tea alcohol (2g/250 mL) which recorded significantly (P<0.05) higher serum albumin.
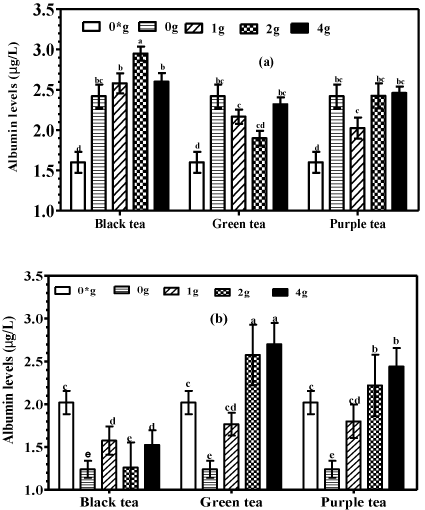
Figure 1: Albumin levels in (a) serum and (b) liver homogenates of mice
supplemented with tea fortified alcoholic beverages 6% (v/v) containing
black, green and purple teas at 0, 1, 2 and 4 g/250mL (m/v) and water only
(0*) for the negative controls.
An evaluation of the effects of plain and tea fortified alcohols on liver albumin was conducted and it showed that supplementation with plain alcohols significantly (P<0.05) reduced liver albumin compared to supplementation with water only (Figure 5B). However, mice supplemented with tea fortified alcohols had significantly higher (P<0.05) liver albumin levels compared to mice supplemented with plain alcohols. Moreover, the group supplemented with black tea alcohol at 2g/250 mL recorded significantly higher (P<0.05) liver albumin compared to water only controls. The results also showed a trend towards increased liver albumin with increased tea concentration. Mice supplemented with green tea at 4 g/250 mL recorded significantly (P<0.05) higher liver albumin compared to mice supplemented at a much lower concentration of 1 g/250 mL.
Effects of tea fortified alcohols on liver alkaline phosphatase (ALP)
Mice supplemented with plain alcohols had significantly (P<0.001) higher liver ALP levels compared to mice supplemented with tea fortified alcohols or water only. Notably, no significant differences (P>0.05) were recorded in liver ALP values between groups supplemented with tea fortified alcohols and water only for the controls (Figure 6).
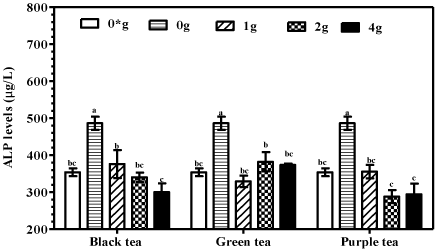
Figure 6: Alkaline phosphatase in liver homogenates of mice supplemented
with tea fortified alcoholic beverages 6% (v/v) containing black, green or
purple at 0, 1, 2 and 4 g/250mL (m/v) and water only (0*) for the negative
controls.
Effects of tea fortified alcohols on glutathione
Serum GSH of the plain and black tea alcohol groups significantly (P<0.001) decreased compared to the control group supplied with water only (Figure 7A). However, mice supplemented with alcohols fortified with green tea at all concentrations and purple tea at 2 g/250 mL recorded significantly higher (P<0.001) serum GSH compared to mice supplied with plain alcohols and water only. Mice supplemented with purple tea alcohols (1 and 4g/250 mL) had serum GSH levels comparable to those for controls of water only (P>0.05).
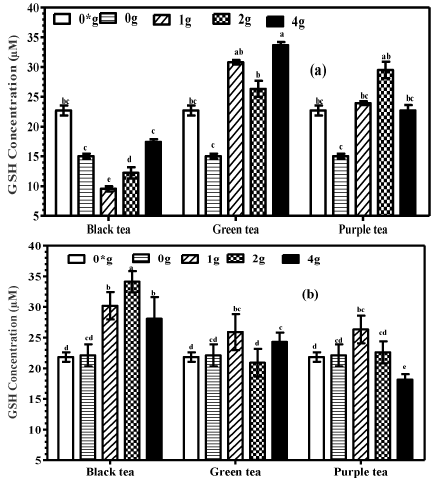
Figure 7: Total GSH levels in (a) serum and (b) liver homogenates of mice
supplemented with tea fortified alcoholic beverages 6% (v/v) containing
black, green or purple teas as base ingredients with 0, 1, 2 and 4 g/250mL
(m/v) and water only (0*) for the negative controls.
Oral administration of black tea alcohols (1 g and 2 g/250 mL) led to a significant increase (P<0.01) in liver GSH compared to animals supplemented with plain alcohols or water only for the controls (Figure 7B). No significant differences (P>0.05) were detected among the other groups.
Discussion
Alcohol, a natural product widely consumed as a social drink, is a common cause of insult to the liver [22]. This is attributed to the fact that almost 80% of alcohol metabolism occurs in the liver as a process of detoxification [23]. Assessment of liver function can be made by estimating the concentration and activities of serum and liver tissue enzymes, an imbalance or altered levels expected during alcohol toxicity [2,24]. This phenomenon was clearly demonstrated in our study by the significant elevation of liver ALP in mice fed on plain alcohols compared to controls on water only. The high levels of ALP can be attributed to malfunctioning of hepatocytes induced by the consumption of alcohol. These findings have been corroborated by previous studies [22-26]. Moreover, administration of plain alcoholic beverages induced oxidative stress as manifested by significant decrease (P<0.05) in serum GSH. Alcohol is metabolized in the liver, first to acetaldehyde and then to acetate by alcohol and aldehyde dehydrogenases respectively [27]. These oxidation reactions are accompanied by the generation of ethyl and hydroxyl ether radicals with concomitant depletion of the body’s supply of antioxidants [1]. Subsequently, this creates an imbalance between oxygen radicals and antioxidants, rendering the body’s supply of antioxidants incapable of detoxifying reactive oxygen species due to excessive production and thereby generating oxidative stress [28]. The research findings inferred that continuous alcohol consumption augmented the generation of free radicals in the experimental mice subsequently leading to the reduction of glutathione content due to increased utilization. This finding agrees with the work of [29] who reported significantly reduced levels of GSH in female rats exposed to ethanol. [2] also reported the ability of ethanol to decrease not only GSH concentrations but also other antioxidants such as vitamins C and E in the liver and kidneys of experimental rats. Other research work also support the fact that oxidative stress plays a pivotal role in the pathogenesis of alcohol toxicity, with the generated free radicals causing damage to liver cells and other vital organs [28]. Therefore, quenching of free radicals is important in providing protection to the liver and other organs against alcohol induced toxicity.
Remarkably, mice fed on tea fortified alcoholic beverages had liver ALP values comparable to those recorded for controls supplied with water only. In addition, the presence of tea Polyphenol in alcoholic beverages prevented to a significant degree a drop in GSH when compared to mice fed plain alcohols. These results point to the potential of tea as a hepatoprotective and antioxidant agent towards alcohol induced toxicity. It is well established that plants portraying good antioxidant capacity are also associated with hepatoprotective potentials [22], and therefore it can be postulated that the protective effects of tea might have been contributed, at least in part, by its high phenolic and flavonoid content. This hypothesis is consistent with previous findings of [1] who reported excellent antioxidant activity from Bauhinia purpurea bark on rats with alcohol induced toxicity [30]. Corroborated these findings and attributed the antioxidant activity of red grape seed extracts to hepatoprotective effects in ethanol-induced cytotoxicity in liver slice cultures. Findings from this study provide new information on the ability of tea (Camellia sinensis) to ameliorate alcohol toxicity and boost antioxidant defenses when used in fortification of alcoholic beverages. However, green and purple teas were more effective in up-regulating serum GSH levels compared to black tea. Similar results were obtained for liver albumin and total protein assays. This observation could be attributed to the variation in the polyphenolic composition of the different teas used in this study. Non-aerated teas contain significantly higher levels of catechins compared to aerated black teas [17,31]. This is because during black tea production, about 75% of catechins in the leaves undergo enzymatic transformation to yield theaflavins and thearubigins, which are less potent antioxidants compared to the catechins [17,32]. Indeed, a previous study established that green tea portrays higher antioxidant activity than black tea, with Epigallocatechin gallate (EGCG) being the most potent catechin and the most potent in antioxidant activity [17]. Therefore the varied effects of the tea fortified alcoholic beverages on GSH albumin and total protein could be attributed to the higher catechin levels in the non-aerated green and purple teas vis-a-vis aerated black teas.
Results obtained in this study also indicate that plain alcohol consumption significantly reduced liver albumin levels in experimental mice. The liver is responsible for alcohol metabolism, and consequently hypoalbuminemia due to continued alcohol consumption is a reflection of hepatocyte injury and poor liver function [26]. Notably, mice fed tea fortified alcohols recorded significantly (P<0.05) increased liver albumin compared to the plain alcohol group. Moreover, serum protein increased in mice supplemented with green and purple tea alcohols vis-à-vis the plain alcohol groups. Increased total protein and albumin level is indicative of improved functional and secretory mechanism of hepatic cells and the hepatoprotective activity of tea (Camellia sinensis). This finding agrees with the work of [7] who reported the ability of Camellia sinensis to enhance total protein and albumin synthesis in male Wistar rats challenged with carbon tetrachloride to induce liver damage. However, mice supplemented with both plain and tea fortified alcohols recorded significantly higher levels (P<0.05) of serum albumin compared to controls fed water only. Alcohol, being an anti-diuretic, is known to cause dehydration by promoting urine production and preventing reabsorption of water by the kidneys [33]. Dehydration in turn causes a decrease in plasma volume, and therefore the values of serum albumin may have been inaccurately amplified in this study.
In addition, when compared to the plain alcoholic beverages, tea fortified alcohols recorded lower PCV levels, the decrease being dependent upon the concentration of tea used to fortify the alcohols. Tea is known to be a potent inhibitor of iron absorption by forming insoluble complexes in the gastrointestinal lumen with concomitant decrease in hemoglobin levels [34,35]. Indeed, findings from a previous study had established that increased consumption of tea is associated with decreased hemoglobin (Hb) concentrations [36]. Moreover, consumption of 20mg of polyphenols from black tea reduced iron absorption in young women in India by as much as 66% in the study by Thankachan [37-39]. Therefore the low PCV levels observed in the tea alcohol groups could be attributed to insufficient Hb in the body causing a reduction in the synthesis of red blood cells.
In conclusion, findings from this study provide further compelling evidence that oxidative stress plays an important role in the pathogenesis of ethanol toxicity. Further, the observation that the presence of tea phenolics in alcoholic beverages ameliorated alcohol-induced decreases in albumin and GSH coupled with the significant decrease in ALP activity suggest the antioxidant and hepatomodulatory properties of tea. Therefore, with validation from higher mammals, tea (Camellia sinensis) may be used as an agent for protection against alcohol toxicity and oxidative stress.
Acknowledgement
The authors would like to thank the National Commission for Science and Technology and Innovation (NACOSTI) and Kenya Agricultural and Livestock Research Organization-Tea Research Institute (KALRO-TRI) for their financial support. The authors also acknowledge Animal Sciences Department of NMK-IPR, for the expert technical input in animal experiments and performing biochemical analyses.
References
- Chaturvedi P, Pipedi-Tshekiso M, Moseki B, Kwape TE. Hepatoprotective potentials of water extract of Bauhinia purpurea bark against alcohol induced toxicity. Scientific Research and Essays. 2011; 6; 4347-4353.
- Esmaeili MA, Sonboli A, Kanani MR, Sadeghi H. Salvia sahendica prevents tissue damages induced by alcohol in oxidative stress conditions: Effect on liver and kidney oxidative parameters. Journal of Medicinal Plants Research. 2009; 3: 276-283.
- Dahiru, D, Obidoa O. Evaluation of the antioxidant effects of Ziziphus mauritiana lam. leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr J Tradit Complement Altern Med. 2008; 5: 39-45.
- Das SK, Vasudevan DM. Alcohol-induced oxidative stress. Life Sci. 2007; 81: 177-87.
- Dubey NK, Kumar R, Tripathi P. Global promotion of herbal medicine: India’s opportunity. Current Science. 2004; 86: 37-41.
- Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant derived polyphenolic flavonoids. Free Radical Research. 1995; 22: 375-383.
- Sengottuvelu S, Duraisami S, Nandhakumar J, Duraisami R, Vasudevan M. Hepatoprotective activity of Camellia sinensis and its possible mechanism of action. Iranian Journal of Pharmacology and Therapeutics. 2008; 7: 9-14.
- Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; 71: 1698S-702S.
- Kerio LC, Bend JR, Wachira FN, Wanyoko JK, Rotich MK. Attenuation of t-Butylhydroperoxide induced oxidative stress in HEK 293 WT cells by tea catechins and anthocyanins. Journal of Toxicology and Environmental Health Sciences. 2011; 3: 367-375.
- Rashid K, Wachira FN, Nyabuga JN, Wanyonyi B, Murilla G, Isaac AO. Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutritional Neuroscience. 2014; 17: 178-185.
- Korir MW, Wachira FN, Wanyoko JK, Ngure RM, Khalid R. The fortification of tea with sweeteners and milk and its effect on in vitro antioxidant potential of tea product and glutathione levels in an animal model. Food Chemistry. 2014; 145: 145-153.
- Ochanda SO, Rashid K, Wanyoko JK, Ngotho M, Faraj AK, Onyango CA, et al. Fortification of alcoholic beverages (12% v/v) with tea (Camellia sinensis) reduces harmful effects of alcohol ingestion and metabolism in mouse model. BMJ Open Gastro 2016; 3: e000058.
- Oyejide OO, Olushola L. Hepatoprotective and antioxidant properties of extract of Carmellia sinensis (black tea) in rats. African Journal of Biotechnology. 2005; 4: 1432-1438.
- El-Beshbishy HA. Hepatoprotective effect of green tea (Camellia sinensis) extract against tamoxifen-induced liver injury in rats. Journal of Biochemistry and Molecular Biology. 2005; 38: 563-570.
- Issabeagloo E, Taghizadieh M. Hepatomodulatory action of camellia sinensis aqueous extract against Isoniazid-Rifampicin combination induced oxidative stress in rat. Advances in Bioresearch. 2012; 3: 18-27.
- Heikal TM, Mossa ATH, Rasoul MAA, Marei GIK. The ameliorating effects of green tea extract against cyromazine and chlorpyrifos induced liver toxicity in male rats. Asian Journal of Pharmaceutical and Clinical Research. 2013; 6; 48-55.
- Karori S, Wachira F, Wanyoko J, Ngure R. Antioxidant capacity of different types of tea products. African Journal of Biotechnology. 2007; 6: 2287-2296.
- Ochanda SO, Wanyoko JK, Onyango CA, Faraj AK, Kamunya SM. Screening of suitable clones for un-aerated tea production. African Journal of Horticultural Science. 2012; 6: 118-134.
- Close B, Banister K, Baumans V, Bernoth EM, Bromage N, Bunyan J, et al. Recommendations for euthanasia of experimental animals: Part 1. Lab Anim. 1996; 30; 293-316.
- Hoff J, Rlagt LV. Methods of blood collection in the mouse. Lab animals. 2000; 29: 47-53.
- Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006; 1: 3159-65.
- Ho WY, Yeap SK, Ho CL, Rahim RA, Alitheen NB. Hepatoprotective Activity of Elephantopus scaber on Alcohol-Induced Liver Damage in Mice. Evidence- Based Complementary and Alternative Medicine. 2012. 2012: 8 Pages.
- Gopumadhavan S, Rafiq M, Azeemuddin M, Mitra SK. Ameliorative effect of Partysmart in rat model of alcoholic liver disease. Indian Journal of Experimental Biology. 2008; 46: 132-137.
- Muthulingam M, Mohandoss P, Indra N, Sethupathy S. Antihepatotoxic efficacy of Indigofera tinctoria (Linn.) on paracetamol induced liver damage in rats. International Journal of Pharmaceutical and Biomedical Research. 2010; 1: 13-18.
- Panda V, Ashar H, Srinath S. Antioxidant and hepatoprotective effect of Garcinia indica fruit rind in ethanol induced hepatic damage in rodents. Interdisciplinary Toxicology. 2012; 5: 207-213.
- Priya N, Venkatalakshmi P. The impact of heavy alcohol consumption and cigarette smoking on liver function-A clinical survey. International Journal of Pharmacy and Pharmaceutical Sciences. 2013; 5: 82-85.
- Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997; 38: 601-628.
- Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003; 27: 277-284.
- Dongare PP, Shah PR, Dhande SR, Kadam VJ. Antihepatotoxic activity of Pogostemon patchouli against alcohol-induced hepatotoxicity in rats. International Journal of Advanced Research. 2013; 1; 225-234.
- Hassan HMM. Hepatoprotective effect of red grape seed extracts against ethanol-induced cytotoxicity. Glob J Biotechnol Biochem. 2012; 7: 30-7.
- Kerio LC, Wachira FN, Wanyoko JK, Rotich MK. Total polyphenols, catechin profiles and antioxidant activity of tea products from purple leaf coloured tea cultivars. Food Chem. 2013; 136: 1405-1413.
- Åuczaj W, Skrzydlewska E. Antioxidative properties of black tea. Prev Med. 2005; 40(6): 910-918.
- Swift R, Davidson D. Alcohol hangover: mechanisms and mediators. Alcohol Health Res World. 1998; 22: 54-60.
- Kaltwasser JP, Werner E, Schalk K, Hansen C, Gottschalk R, Seidl C. Clinical trial on the effect of regular tea drinking on iron accumulation in genetic haemochromatosis. Gut. 1998; 43: 699-704.
- Hunt JR, Roughead ZK. Adaptation of iron absorption in men consuming diets with high or low iron bioavailability. American Journal of Clinical Nutrition. 2000; 71: 94-102.
- Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ. 1995; 310: 693-696.
- Thankachan P, Walczyk T, Muthayya S, Kurpad AV, Hurrell RF. Iron absorption in young Indian women: The interaction of iron status with the influence of tea and ascorbic acid. American Journal of Clinical Nutrition. 2008; 87: 881-886.
- Dubey NK, Kumar R, Tripathi P. Global promotion of herbal medicine: India’s opportunity. Current Science. 2004; 86: 37-41.
- Issabeagloo E, Ahmadpoor F, Kermanizadeh P, Taghizadieh M. Hepatoprotective effect of green tea on hepatic injury due to Leflunomide in rat. Asian Journal of Experimental Biological Sciences. 2012; 3: 136-141.
Citation: Ochanda SO, Rashid K, Ngotho M, Faraj AK, Wanyoko JK, Onyango CA, et al. Investigations of Antioxidant and Hepatomodulatory Potentials of Tea (Camellia sinensis) in Black, Green and Purple Tea Fortified Alcoholic Beverages (6% V/V) Using a Mouse Model. Austin J Gastroenterol. 2017; 4(4): 1092.