
Case Report
Austin J Gastroenterol. 2019; 6(1): 1098.
The Smallest Solitary Fibrous Tumor of the Stomach Reported
Al-Zubaidi AM1*, Alqannas MH2 and Bashanfer GA3
¹Department of Medicine, Endoscopy Unit, King Khalid Hospital, Najran 1120, Saudi Arabia
²Department of Surgery, Bariatric Surgery, King Khalid Hospital, Najran 1120, Saudi Arabia
³Department of Laboratory, Histopathology Section, King Khalid Hospital, Najran 1120, Saudi Arabia
*Corresponding author: Al-Zubaidi AM, Department of Medicine, Endoscopy Unit, King Khalid Hospital, Najran 1120, Saudi Arabia
Received: March 08, 2019; Accepted: April 15, 2019; Published: April 22, 2019
Abstract
Solitary fibrous tumors are spindle-cell neoplasms that usually originate in the pleura and peritoneum, and rarely in the stomach. To our knowledge, there is only 6 case reporting a solitary fibrous tumor arising from the stomach in the English literature. Here we report the case of a 35-year-old man with a small solitary fibrous tumor arising from the stomach greater curvature found incidentally during diagnostic gastroscopy. A solitary fibrous tumor arising from the stomach, although very rare, should be considered as a diagnostic possibility for any gastric submucosal tumors.
Keywords: Solitary Fibrous Tumor; Stomach; CT Findings
Introduction
Solitary Fibrous Tumor (SFTs) are rare and represent 2% of all soft tissue tumor [1]. It is commonly found in the pleura, only 0.6% of these tumors are extrapleural and can manifest in the abdomen, Esophagus, stomach, pancreases, rectum, etc [2-5]. The first case of a pleural solitary fibrous tumor was reported by Klemperer at 1931 [6]. Approximately 12-22% of SFTs are found to be malignant; however, the histological definitions that suggest a tendency toward malignancy are not well-defined [7]. Fibrous tumor of the stomach is very rare and only six cases reported until March 2017.
Case Presentation
A 38-year-old man was referred from gastroenterology clinic for upper and lower endoscopy with a symptom of dyspepsia and epigastric discomfort did not respond to PPI, bleeding per rectum with alerted bowel habit. The patient had a history of nephrotic syndrome 18 years ago with biopsy-proven membranous proliferative glomerulonephritis. His physical examination was unremarkable as well as he has normal Initial biochemical and hematological laboratory studies.
Esophago Gastro Duodenoscopy (EGD) showed small 6mm nodule in the antrum along the greater curvature with normal overlying mucosa, which is mobile to touch by biopsy forceps (Figure 1).
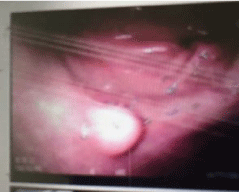
Figure 1: Endoscopic Image; show small submucosal anatral lesion.
During endoscopy, the lesion elevated by injection with saline, snared in a block using the regular snare and sent for histopathology, the procedure went uneventful and patient discharge home in the same days on omeprazole tab 20mg daily.
Histopathology of the resected specimen show polypoid piece lined by antral type mucosa. The subepithelium shows a tumor (4mm) composed of a proliferation of bland oval to spindle cells embedded in fibromyxoid stroma Histopathology image [1] (Figure 2). Crushed cells also are seen. The tumor cells are diffusely positive for vimentin and CD34 and BCL2 immunostains Histopathology (Figure 3,4) and weekly positive for CD99. The tumor cells are negative for CK AE1/ AE3, S-100, SMA, CD117, DOG-I caldesmon, B-Catenin, ALK and HHV8. These findings were consistent with the diagnosis of a Gastric Solitary Fibrous Tumor (Gastric SFT). No Mitosis, cellular atypia or necrosis No H. pylori microorganisms were seen.
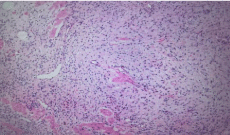
Figure 2: Histopatology image. Bland spindle cells embedded in collagenous
stroma.

Figure 3: Histopatology image. The tumor cells are diffusely positive for
CD34 immunostains.
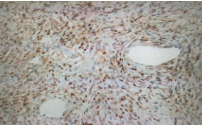
Figure 4: Histopatology image. The tumor cells are diffusely positive for
BCL2 immunostains.
The patient was immediately informed about the diagnosis and follow up endoscopy done after six months which show depresses scare at the site of the lesion with no evidence of recurrence as in Endoscopy image [2] (Figure 5). Extensive biopsy from the site was taken and came negative for recurrence (Figure 5).
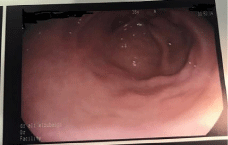
Figure 5: Endoscopic image. Show scare at the site of removed tumor.
Abdominal and chest CT was done which show normal gastric wall with no evidence of any lesion in pleura.
Patient educated regarding the long-term follow-up of her lesion.
A follow-up, the patient at 6 months show clinically improvement and PPI was tapered off.
Discussion
Solitary Fibrous Tumors are rare, mesenchymal neoplasms described initially by Klemperer and Rabin at 1931 who reported 5 cases of primary pleural neoplasms proposed that SFT was of submesothelial origin [6] but later on, it has been reported in extrapleural locations such as the pelvis, abdomen, retroperitoneum, buccal space, maxillary sinus, liver, pancreas, suprarenal region, and kidneys [7,8]. Although the true incidence of pleural SFT is unknown, it has been estimated to occur with a frequency of 2.8 per hundred thousand individual [9] it affects both genders equally and frequently found in middle age people [10]. The symptoms of SFTs usually nonspecific and varied according to the size and location [11].
Gastric SFT is a rare clinicopathologic entity. To our knowledge, only 6 cases of gastric SFTs have been reported to date [11-17]. Three patients with gastric SFTs were male and four were female, with a median age of 53.4 years (26 -77 year) at the time of diagnosis and a median tumor size of 3.37cm (0.5 to 8.5 cm) (Table 1).
Age
Sex
Size of tumor
77Y
Female
3CM
70Y
Male
8.5cm
26 Y
Male
5.4cm
43Y
2.7cm
65Y
Female
2.5cm
55Y
Female
7cm
Table 1:
The diagnosed in four cases include our case was incidentally. Patients with large intra-abdominal SFT usually symptomatic and present with; palpable mass, abdominal pain, vomiting or weight loss. However, incidental SFTs, as in our case, small tumors are typically asymptomatic [11-17]. The differential diagnosis of gastric SFT includes many gastrointestinal stromal tumors like; leiomyosarcoma, calcifying fibrous tumor, leiomyoma, gastric carcinoid fibrosarcoma, malignant fibrous histiocytoma, and malignant mesenchymoma etc [8,9,11].
Although there are no specific radiological features for SFTs, CT and MRI are frequently used in the diagnosis of SFT [9]. On MRI SFTs typically display low T1 signal intensity and variable T2 signal intensity, and they enhance intensely after intravenous gadolinium administration, while on CT the lesion often appears homogenous with enhancement after contrast administration [8,9,18].
Needle aspiration biopsy for SFTs usually provides inconclusive results. However, histopathologic and immunohistochemistry findings are key to establish a definitive diagnosis as the tumor commonly Express CD34, BCL2, CD99 and STAT-6 and Negative for S-100 and Epithelial Membrane Antigen (EMA) [12]. Diagnosis of gastric SFTs was mostly established based on the combination of clinical suspicion, imaging findings, and histopathology, and was confirmed with immunohistochemical staining. In our review of gastric SFTs cases, all 6 tumors were positive for CD34 and 3 were positive for vimentin our case was the 7th was positive for, Vimentin, CD34 .BCL2 an Negative for CK AE1/AE3, S-100, SMA, Desmin, CD117, DOG-I, B-Catenin, ALK, and HHV8 immunostains. For CK AE1/AE3, S-100 and Epithelial Membrane Antigen (EMA).
Complete en block surgical resection with negative margin is the mainstay of treatment of localized SFT lesions even for high-risk tumors, given the low overall metastatic potential [19] there is some promise of the vascular endothelial growth factor inhibitors and other tyrosine kinase-inhibiting molecular agents such as bevacizumab, sunitinib, pazopanib, and sorafenib for therapy of advanced SFT [20].
Our patient’s gastric SFTs were treated with Endoscopic Submucosal Resection (EMR) the patients showed a cure with no recurrence on follow-up. Therefore, to our knowledge, our case represents the first report of an exceptionally the smallest gastric SFT incidentally discovered during endoscopy, confirmed by histopathology study, and successfully treated with EMR. Hence, curative endoscopic resection should be recommended in patients with small gastric SFT.
The possibility of gastric SFT should be always considered when an exceptionally small subepithelial nodule is seen during routine endoscopy. The unpredictable biological behavior and the possibility of recurrence, with no stander guidelines for follow up a long-term clinical and endoscopic follow-up may be necessary at the present time.
References
- Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002; 94: 1057-1068.
- Van Houdt WJ, Westerveld CM, Vrijenhoek JE, van Gorp J, van Coevorden F, Verhoef C, et al. Prognosis of solitary fibrous tumors: A multicenter study. Ann SurgOncol. 2013; 20: 4090-4095.
- Andrew R Baxter, Elliot Newman, Cristina H Hajdu. Solitary fibrous tumor of the pancreas. J Surg Case Rep. 2015; 2015: rjv144.
- Xue-Ming Li, Jing Reng, Peng Zhou, Ying Cao, Zhu-Zhong Cheng, Yan Xiao, et al. Solitary fibrous tumors in abdomen and pelvis: Imaging characteristics and radiologic-pathologic correlation. World J Gastroenterol. 2014; 20: 5066- 5073.
- Park SB, Park YS, Kim JK, Kim MH, Oh YT, Kim KA, et al. Solitary fibrous tumor of the genitourinary tract. AJR Am J Roentgenol. 2011; 196: W132-W137.
- Klemperer P, Rabin CB. Primary neoplasms of the pleura: A report of five cases. Arch Patho. 1931; 11: 385-412.
- Robinson LA. Solitary fibrous tumor of the pleura. Cancer Control. 2006; 13: 264-269.
- Chick JF, Chauhan NR, Madan R. Solitary fibrous tumors of the thorax: Nomenclature, epidemiology, radiologic and pathologic findings, differential diagnoses, and management. AJR Am J Roentgenol. 2013; 200: W238.
- Keraliya AR, Tirumani SH, Shinagare AB, Zaheer A, Ramaiya NH. Solitary fibrous tumors: 2016 imaging update. RadiolClin North Am. 2016; 54: 565- 579.
- Dango S, Kirschaum B, Passlick B. Solitary fibroma of the pleura: clinical findings and prognosis. ZentralblChir. 2008; 133: 227-230.
- Ge W, Yu DC, Chen G, et al. Clinical analysis of 47 cases of solitary fibrous tumor. OncolLett. 2016; 12: 2475-2580.
- Faisal Inayat, QulsoomHussain, KhurramShafique, Abu Hurairah, Evan B. Grossman. Solitary Fibrous Tumor of the Stomach. ACG Case Rep J. 2017; 4: e35.
- Nabeshima K, Tomioku M, Nakamura K, Yasuda S. Solitary fibrous tumor of the stomach treated with laparoscopic and endoscopic cooperative surgery. Tokai J ExpClin Med. 2015; 40: 120-123.
- Park SH, Kim MJ, Kwon J, Park JP, Park MS, Lim JS, et al. Solitary fibrous tumor arising from stomach: CT findings. Yonsei Med J. 2007; 48: 1056-1060.
- Lee WA, Lee MK, Jeen YM, Kie JH, Chung JJ, Yun SH, et al. Solitary fibrous tumor arising in gastric serosa. Pathol Int. 2004; 54: 436-439.
- Boskovic T, Zivojinov M, Sabo JI, Budakov Z, Veljkovic R, Zivojinov S, et al. Rare solitary fibrous tumor of the stomach: A case report. Vojnosanit Pregl. 2015; 72: 1035-1038.
- Shidham VB, Weiss JP, Quinn TJ, Grotkowski CE. Fine needle aspiration cytology of gastric solitary fibrous tumor: A case report. ActaCytol. 1998; 42: 1159-1166.
- Vaz Salgado MA, Soto M, Reguero ME, Muñoz G, Cabañero A, Gallego I, et al. Clinical behavior of solitary fibrous tumor: A retrospective review of 30 patients. Clin Transl Oncol. 2017; 19: 357-363.
- O’Neill AC, Tirumani SH, Do WS, Keraliya AR, Hornick JL, Shinagare AB, et al. Metastatic patterns of solitary fibrous tumors: a single-institution experience. AJR Am J Roentgenol. 2017; 208: 2-9.
- Stacchiotti S, Negri T, Libertini M, Palassini E, Marrari A, De Troia B, et al. Sunitinib malate in solitary fibrous tumor (SFT). Ann Oncol. 2012; 23: 3171- 3179.