
Research Article
Austin J Gastroenterol. 2019; 6(2): 1105.
Clif-Sofa is Superior to Other Liver-Specific Scores for Predicting Mortality in Acute-on-Chronic Liver Failure and Decompensated Cirrhosis
Grochot RM1, Luz LB2, Garcia R3, Balbinot RA4, Balbinot SS5 and Soldera J6*
1Physician, Internal Medicine, Hospital Virvi Ramos, Residency in Oncology, Hospital Geral, Caxias do Sul (RS), Brazil
2Physician, Universidade de Caxias do Sul (UCS), Caxias do Sul (RS), Brazil
3Medical student, Universidade de Caxias do Sul (UCS), Caxias do Sul (RS), Brazil
4Physician, Titular Professor of Gastroenterology and Hepatology, Universidade de Caxias do Sul (UCS), Caxias do Sul (RS). Doctorate in Gastroenterology, Department of Gastroenterology, Universidade de Sao Paulo (USP), Sao Paulo (SP), Brazil
5Physician, Titular Professor of Gastroenterology and Hepatology, Universidade de Caxias do Sul (UCS), Caxias do Sul (RS). Doctorate in Gastroenterology, Department of Gastroenterology, Universidade de São Paulo (USP), São Paulo (SP), Brazil
6Associate Professor of Gastroenterology and Hepatology, Universidade de Caxias do Sul (UCS), Caxias do Sul (RS). Master’s in Medicine: Hepatology, Department of Hepatology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre (RS), Brazil
*Corresponding author: Jonathan Soldera, Associate Professor of Gastroenterology and Hepatology, Universidade de Caxias do Sul (UCS), Caxias do Sul (RS). Master’s in Medicine: Hepatology, Department of Hepatology, Universidade Federal de Ciências da Saúde de Porto Alegre (UFCSPA), Porto Alegre (RS), Brazil
Received: October 31, 2019; Accepted: December 03, 2019; Published: December 10, 2019
Abstract
Background: A particular clinical entity, acute-on-chronic liver failure (ACLF), defined as organ failures associated with decompensated cirrhosis carries a higher risk of death in the short term. Liver-specific scores have been developed to predict mortality in such population in previous studies.
Design and Setting: Historical cohort study conducted in a mixed public and private tertiary care teaching hospital.
Methods: Data from medical records from January 2013 to December 2014 were obtained by searching the hospital electronic database for codes associated to liver disease. Paper medical charts were hand-analyzed. Liverspecific scores were calculated and ROC-curves pairwise comparisons were performed using DeLong test.
Results: CLIF-SOFA was able to predict mortality in 28, 90 and 365-day, with AUROC of 0.71, 0.75 and 0.66, respectively. CLIF-SOFA was superior to CLIF-C AD/ACLF, MELD and MELD-Na in prediction of 90-day mortality (p ‹ 0,05). Values of CLIF-SOFA above 11 was able to predict higher mortality for all patients, with sensitivity of 64%, 50% and 47% and specificity of 72%, 89% and 82% for 28, 90 and 365-day mortality, respectively (p ‹ 0,05).
Conclusion: CLIF-SOFA score was superior to other liver-specific scores for predicting mortality in a cohort of a mixed public and private teaching hospital in Brazil, especially in values above 11.
Keywords: Liver cirrhosis; End stage liver disease; Organ dysfunction scores; Prognosis; Cohort studies
Introduction
Chronic liver injury leads to fibrosis and nodular regeneration, which culminates into cirrhosis, a mostly irreversible condition [1]. Such injuries might be caused by several conditions, as viral hepatitis, alcoholic liver disease and non-alcoholic fatty liver disease. The natural history of cirrhosis, after it is established, is marked by the progression from compensated cirrhosis to decompensated cirrhosis (DC). Cirrhosis is the 9th leading cause of death in the West in 2015, according to World Health Organization [2].
DC is the principal cause of hospital admittance in cirrhotic patients [3-7]. Nevertheless, in the last decade, it has been observed that the association of organ failures to DC might increase mortality independently [8-12]. Therefore, a particular clinical entity, acute-onchronic liver failure (ACLF), has been suggested as the responsible for such increase in mortality [10-13]. Score systems were needed to differentiate between DC and ACLF, defining and staging it. The importance of such resides in the fact that while DC translates into progression of the disease, ACLF is an acute event, potentially reversible, with a high mortality [14,15].
Organ failure-associated scores, initially developed for intensive care, have been shown to be better predictors of ACLF-related mortality than classic liver-specific scores. Mortality for cirrhotic patients admitted in the intensive care unit (ICU) with three or more organ failures has been shown to be as high as 70% in the first day of admission, increasing to around 89% by the third day [15,16]. This perception of ACLF has been profoundly shifted by the publication of the CANONIC study, a prospective cohort study published in 2013, which translated a score commonly used in the ICU to the ACLF setting, creating the CLIF-SOFA (Chronic Liver Failure Sequential Organ Failure Assessment) and dividing ACLF into three categories with distinct mortality [17]. Therefore, understanding ACLF has become paramount in order to better understand the gap between DC and death.
The purpose of this paper is to analyze the accuracy of CLIF SOFA to predict mortality and compare it to other liver-specific scores in the CD and ACLF setting, in a mixed public and private tertiary care teaching hospital in Brazil.
Methods
Study population
The study was approved by the research ethics committee of the hospital on October 20, 2014, under protocol no. 35359813.4.0000.5523. A historical cohort study was conducted, analyzing data from hospital charts from January 2013 to December 2014. Patients were found by searching through a mixed public and private teaching hospital electronic database for International Classification of Diseases (ICD-10) codes F10, K70, K70.1, K70.2, K70.3, K71.7, K74, K74.2, K74.3, K74.6, K77. Paper medical charts were hand-analyzed. Patients over 18 years old with laboratory and imaging data supporting the diagnosis of cirrhosis were included. Patients were excluded if they did not have a diagnosis of cirrhosis when the chart was reviewed or had incomplete charts. Data regarding clinical and laboratory variables were gathered and liverspecific scores were calculated.
Variables
Clinical and laboratorial variables were gathered by analyzing paper medical charts and electronic laboratory data. Laboratory data is expressed in units used in the hospital. Diagnosis of hepatocellular carcinoma (HCC) was made using standardized imaging techniques [18]. Diagnosis of Hepatorenal Syndrome were made using the previously published criteria for diagnosis [19]. Diagnosis of infection was made through a positive culture or neutrophil count in ascitis [20].
MELD and MELD-Na
MELD (Model for End-Stage Liver Disease) [21] and MELD-Na (MELD-Sodium) [22] are scores used to predict 90-day mortality and are currently used for organ allocation in liver transplantation. They were calculated using an online calculator (MELD: https://www. mdcalc.com/meld-score-model-end-stage-liver-disease-12-older) (MELD-Na: https://www.mdcalc.com/meldna-meld-na-score-livercirrhosis).
CLIF-SOFA
CLIF-SOFA is an adaptation of the SOFA score commonly used in the intensive care setting, developed by the CANONIC group in 2013 and further validated [17] It was calculated using an online calculator developed by the CLIF Research Group (https://www. clifresearch.com/ToolsCalculators.aspx).
ACLF grade
The CLIF-SOFA score is also used to analyze organ failures and define the presence of ACLF and its grade [17]. It was calculated using an online calculator developed by the CLIF Research Group (https:// www.clifresearch.com/ToolsCalculators.aspx).
CLIF-C AD/ACLF
CLIF Consortium Acute Decompensation (CLIF-C AD) score and CLIF-C ACLF are scores also developed by the CANONIC group that predict expected mortality for 30-day, 90-day, 180-day and 365- day for DC and ACLF patients [23]. They were calculated using an online calculator developed by the CLIF Research Group (https:// www.clifresearch.com/ToolsCalculators.aspx). The online calculator, after the result of the presence of ACLF and the value of CLIF-SOFA, automatically analyzes if CLIF-C AD or ACLF applies in each case and calculates accordingly.
Outcome
Outcome data regarding survival were gathered using hospital charts and searching through national death databases (https://www. falecidosnobrasil.org.br/). If the patient had more than one hospital admission, data regarding only the first were collected.
Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 15.0 and MedCalc. Categorical variables are described using frequency and continuous variables by mean and standard deviation. ROC-curves were generated to analyze sensitivity and specificity of the scores and pairwise comparisons were performed using DeLong test in order to compare scores. Younden index was used to generate ideal cut-off values, sensitivity and specificity.
Results
Electronic ICD search retrieved 190 hospital admissions. Of these, 131 admissions were excluded due to the other diagnosis for admission than cirrhosis; and 8 admissions were excluded for being for the same patients. After chart analysis, 51 patients in their first hospital admission in the analyzed period were included in the study. Demographic, clinical and laboratorial data are described in Table 1 for the study population and for either DC or ACLF. DC was present in 33 patients, while ACLF in 18 patients.
Variable
Study population
Decompensated Cirrhosis
Acute-on-chronic liver failure
(n = 51)
(n = 33)
(n = 18)
Age (years)*
53 (11)
54 (11)
51 (12)
Leukocytes (10³/mm³)*
8,8 (4,8)
8.1 (4.1)
10.3 (5.9)
Platelets (10³/mm³)*
134 (93)
155 (98)
97(71)
BMI (kg/m²)*
25 (3.6)
25 (3.9)
25 (2.9)
MBP (mmHg)*
75 (14)
75 (15)
75 (14)
Creatinine (mg/dL)*
1.59 (1.1)
0.97 (0.17)
2.7 (1.3)
Urea (mg/dL)*
48 (37)
35 (24)
72 (45)
Sodium (mmol/L)*
137 (5.2)
137 (5.2)
135 (5.2)
PaO2 (mmHg)*
85 (26)
84 (30)
85 (17.2)
AST (U/L)*
125 (157)
104 (103)
163 (223)
ALT (U/L)*
137 (303)
77 (110)
248 (477)
GGT (U/L)*
541 (729)
498 (582)
620 (956)
Alkaline phosphatase (U/L)*
220 (137)
243 (148)
177 (104)
Albumin (g/dL)*
2.0 (1.0)
1.9 (1.7)
2.1 (0.5)
INR*
1.5 (0.6)
1.3 (0.4)
1.8 (0.7)
Bilirubin (mg/dL)*
Total
2.4 (2.1)
2.1 (2.1)
2.9 (2.2)
Direct
1.3 (1.6)
1 (1.5)
1.7 (1.8)
Liver scores*
MELD
15.2 (9.7)
10.4 (5.6)
23.9 (9.6)
MELD-Na
16.6 (10.4)
11.9 (6.7)
25.3 (10.4)
CLIF-SOFA
10.9 (2.4)
10.3 (2.1)
11.9 (2.6)
CLIF-C AD/ACLF
54 (11.5)
49.7 (8.5)
63.1 (11.2)
Sex**
Male
42 (82)
25 (76)
17 (94)
Female
9 (18)
8 (24)
1 (6)
Health Service**
Public
46 (90)
30 (90)
16 (89)
Private
5 (10)
3 (10)
2 (11)
Etiology**
Alcohol
46 (90)
29 (88)
17 (94)
Other
5 (10)
4 (12)
1 (6)
Virus**
Hepatitis B
1 (2)
1 (3)
0
Hepatitis C
9 (18)
6 (18)
3 (17)
HIV
1 (2)
0
1 (6)
Hepatocellular carcinoma**
Yes
5 (10)
4 (12)
1 (6)
No
46 (90)
29 (88)
17 (94)
Hepatorenal syndrome**
Yes
6 (12)
1 (3)
5 (28)
No
45 (88)
32 (97)
13 (72)
Infection**
SBP
6 (12)
2 (6)
4 (22)
UTI
30 (59)
20 (61)
10 (56)
RTI
6 (12)
3 (9)
3 (16)
Other
3 (5)
2 (7)
1 (6)
None
6 (12)
6 (17)
0
Hepatic encephalopathy**
Absent
26 (51)
19 (58)
7 (40)
Present
25 (49)
14 (42)
11 (60)
Grade I
12 (23)
8 (24)
4 (22)
Grade II
4 (8)
2 (6)
2 (11)
Grade III
1 (2)
0
1 (5)
Grade IV
8 (16)
4 (12)
4 (22)
Survival**
28-day
37 (72)
26 (78)
11 (61)
90-day
29 (57)
23 (69)
6 (33)
365-day
16 (31)
13 (39)
3 (17)
BMI: Body Mass Index; MBP: Mean Blood Pressure; AST: Aspartate Transaminase; ALT: Alanine Transaminase; GGT: Gamma-Glutamyl Transferase; INR: International Normalized Ratio; MELD: Model For End-Stage Liver Disease; MELD-Na: Modified Model Including Sodium; CLIF-SOFA: Chronic Liver Failure Sequential Organ Failure Assessment; CLIF-C AD/ACLF: CLIF Consortium Acute Decompensation/Acute-On-Chronic Liver Failure; SBP: Spontaneous Bacterial Peritonitis; UTI: Urinary Tract Infection; RTI: Respiratory Tract Infection.
*Mean (standard deviation); **Frequency (%).
Table 1: Demographic, clinical and laboratory findings of the study population and for each acute-on-chronic liver failure (ACLF) grade.
Analysis comparing the scores CLIF-C AD and CLIF-C ACLF, CLIF-SOFA, MELD and MELD-Na were made for 28, 90 and 365-day survival. Table 2 presents the data regarding AUROC comparisons for all patients, showing superiority for CLIF-SOFA score over other scores (Figure 1). Table 3 presents the data regarding AUROC comparisons for DC patients (Figure 2) and Table 4 for ACLF patients (Figures 3), showing discrete or absent superiority for CLIF-SOFA score over the other liver-specific scores when patients were stratified to either DC or ACLF. CLIF-SOFA was able to predict mortality in 28, 90 and 365-day, with AUROC of 0.71, 0.75 and 0.66, respectively. CLIF-SOFA was superior to CLIF-C AD/ACLF, MELD and MELD-Na in prediction of 90-day mortality (p ‹ 0,05).
Variable
AUROC (95% CI)
p value (vs. CLIF-SOFA)
28-day
90-day
365-day
CLIF-SOFA
0.71 (0.57-0.83)
0.75 (0.61-0.86)
0.66 (0.52-0.79)
CLIF-C AD/ACLF
0.52 (0.38-0.67)
0.51 (0.36-0.65)
0.56 (0.41-0.69)
p=0.11
p=0.01
p=0.36
MELD
0.54 (0.39-0.68)
0.50 (0.36-0.65)
0.52 (0.38-0.66)
p=0.11
p=0.05
p=0.21
MELD-Na
0.57 (0.41-0.71)
0.54 (0.40-0.68)
0.55 (0.40-0.69)
p=0.16
p=0.02
p=0.29
AUROC: Area under the Receiver Operator Curve; MELD: Model for End-Stage Liver Disease; MELD-Na: Modified Model Including Sodium; CLIF-SOFA: Chronic Liver Failure Sequential Organ Failure Assessment; CLIF-C AD/ACLF: CLIF Consortium Acute Decompensation/acute-on-chronic liver failure; DC: Decompensated cirrhosis.
Table 2: AUROC for DC and ACLF patients.
Variable
AUROC (95% CI)
p value (vs. CLIF-SOFA)
28-day
90-day
365-day
CLIF-SOFA
0.61 (0.43-0.88)
0.67 (0.49-0.83)
0.50 (0.32-0.68)
CLIF-C AD
0.53 (0.34-0.70)
0.59 (0.40-0.76)
0.50 (0.32-0.68)
p=0.66
p=0.59
p=0.96
MELD
0.56 (0.38-0.73)
0.70 (0.52-0.85)
0.60 (0.42-0.77)
p=0.78
p=0.87
p=0.43
MELD-Na
0.57 (0.38-0.74)
0.54 (0.36-0.72)
0.52 (0.34-0.70)
p=0.72
p=0.46
p=0.86
AUROC = Area under the Receiver Operator Curve; MELD = Model for End-Stage Liver Disease; MELD-Na =
Modified Model Including Sodium; CLIF-SOFA = Chronic Liver Failure Sequential Organ Failure Assessment; CLIF-C AD = CLIF Consortium Acute Decompensation; DC = Decompensated cirrhosis.
Table 3: AUROC for DC patients.
Variable
AUROC (95% CI)
p value (vs. CLIF-SOFA)
28-day
90-day
365-day
CLIF-SOFA
0.76 (0.81-0.92)
0.76 (0.50-0.92)
0.92 (0.69-0.99)
CLIF-C ACLF
0.60 (0.35-0.82)
0.69 (0.43-0.88)
0.57 (0.32-0.80)
p=0.41
p=0.48
p=0.26
MELD
0.53 (0.28-0.76)
0.61 (0.35-0.82)
0.53 (0.28-0.76)
p=0.22
p=0.48
p=0.07
MELD-Na
0.51 (0.27-0.75)
0.61 (0.35-0.82)
0.55 (0.30-0.78)
p=0.23
p=0.48
p=0.09
AUROC: Area under the Receiver Operator Curve; MELD: Model for End-Stage Liver Disease; MELD-Na: Modified Model Including Sodium; CLIF-SOFA: Chronic Liver Failure Sequential Organ Failure Assessment; LIF-C AD: CLIF Consortium acute-on-chronic liver failure; ACLF: acute-on-chronic liver failure.
Table 4: AUROC for ACLF patients.
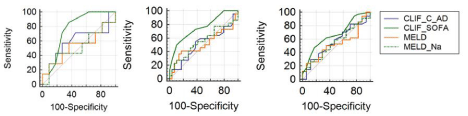
Figure 1: ROC curves for 28, 90 and 365-day survival for all patients.
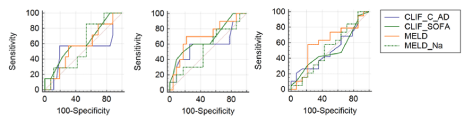
Figure 2: ROC curves for 28, 90 and 365-day survival for DC patients.
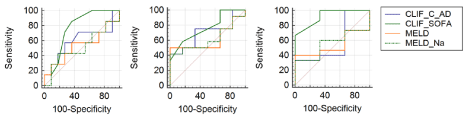
Figure 3: ROC curves for 28, 90 and 365-day survival for ACLF patients.
Table 5 shows the ideal cut-off for predicting mortality using the ROC curve for each score and time frame, for all patients, DC and ACLF patients. Values of CLIF-SOFA above 11 was able to predict higher mortality for all patients, with sensitivity of 64%, 50% and 47% and specificity of 72%, 89% and 82% for 28, 90 and 365-day mortality, respectively (p ‹ 0,05). CLIF-SOFA was able to predict mortality in every time frame for all patients and ACLF patients. CLIF-SOFA values above 11 were associated to higher mortality.
Variable
Survival - hazard ratio (95% CI)
28-day
90-day
365-day
CLIF-SOFA
All patients
> 11
> 12
> 11
Sn 64% Sp 72%
Sn 50% Sp 89%
Sn 47% Sp 82%
p < 0.01
p < 0.01
p = 0.03
ACLF patients
> 11
> 12
> 8
Sn 85% Sp 63%
Sn 58% Sp 83%
Sn 100% Sp 66%
p = 0.01
p = 0.02
p <0.01
DC patients
> 10
> 11
> 9
Sn 57% Sp 65%
Sn 50% Sp 82%
Sn 47% Sp 35%
p = 0.34
p = 0.1
p = 0.9
CLIF-C AD/ACLF
All patients
> 54
> 54
> 62
Sn 64% Sp 62%
Sn 54% Sp 62%
Sn 29% Sp 88%
p = 0.76
p = 0.89
p = 0.48
ACLF patients
> 61
> 56
> 56
Sn 57% Sp 72%
Sn 41% Sp 100%
Sn 33% Sp 100%
p = 0.51
p = 0.13
p = 0.73
DC patients
> 54
> 46
> 39
Sn 57% Sp 80%
Sn 60% Sp 73%
Sn 21% Sp 92%
p = 0.84
p = 0.45
p = 0.94
MELD
All patients
> 27
> 8
> 8
Sn 28% Sp 89%
Sn 40% Sp 82%
Sn 61% Sp 17%
p = 0.67
p = 0.91
p = 0.77
ACLF patients
> 27
> 21
> 21
Sn 57% Sp 63%
Sn 50% Sp 100%
Sn 40% Sp 100%
p = 0.84
p = 0.43
p = 0.82
DC patients
> 8
> 8
> 8
Sn 57% Sp 69%
Sn 70% Sp 78%
Sn 57% Sp 78%
p = 0.6
p = 0.06
p = 0.32
MELD-Na
All patients
> 8
> 27
> 26
Sn 85% Sp 35%
Sn 27% Sp 89%
Sn 26% Sp 88%
p = 0.41
p = 0.58
p = 0.55
ACLF patients
> 21
> 21
> 21
Sn 42% Sp 81%
Sn 41% Sp 100%
Sn 33% Sp 100%
p = 0.9
p = 0.42
p = 0.72
DC patients
> 8
> 15
> 10
Sn 85% Sp 46%
Sn 80% Sp 39%
Sn 57% Sp 57%
p = 0.54
p = 0.67
p = 0.8
MELD: Model for End-Stage Liver Disease; MELD-Na: Modified Model Including Sodium; CLIF-SOFA: Chronic Liver Failure Sequential Organ Failure Assessment; CLIF-C AD/ACLF: CLIF Consortium Acute Decompensation/acute-on-chronic liver failure; ACLF: Acute-on-chronic liver failure; DC: decompensated cirrhosis; Sn: Sensitivity; Sp: Specificity.
Table 5: Ideal cut-offs for each score and time interval.
Discussion
The definition of ACLF has been improved ever since the first supplement dedicated to this subject [9,24-29] integrating intensive care and Hepatology. This was largely due to the publication of CANONIC study, responsible for the current definition of this entity in the West [17]. A different set of criteria developed by the Asia- Pacific Association for the Study of the Liver has been described, but it appears to be inferior to the one developed by the EASL-CLIF [30,31].
In the past couple of decades, several studies have been published regarding the clinical nature of ACLF, but they have been undermined because of the lack of a consensual definition of ACLF. In this study, we analyzed the role of the definitions and scores proposed by the EASL-CLIF (European Association for the Study of the Liver - Chronic Liver Failure) consortium, comparing their accuracy for survival [17].
In this study, the prevalence of ACLF was 35.3% (grade 1 in 13.7%, grade 2 in 19.6% and grade 3 in 2%). These results are not very close to those obtained in the CANONIC study, with an ACLF prevalence of 22.6% [17], or in a similar Brazilian study, with a prevalence of 24% [32], or in a similar North-American study, with a prevalence of 26.4% [33], where grade 1 was 11.0%, 17.7% and 12.8%, respectively.
The ACLF group showed a 28-day mortality of 39% (29, 40 and 100% in ACLF grades 1, 2 and 3, respectively), compared to 22% in non-ACLF patients. In the CANONIC study, 28-day mortality was 33.9%, significantly lower than that reported here [17], whereas the Brazilian study showed very similar mortality rates of 39% [32].
The superiority of CLIF-SOFA has been demonstrated for shortterm for alcoholic cirrhosis in a previous study, in comparison to other liver-specific scores [33]. It has been demonstrated to be superior to CLIF-C AD/ACLF, MELD and MELD-Na in other previous study, even for extra-hepatic insults [34]. It has been studied even in alcoholic hepatitis, showing superiority to even scores that are applied specifically for this condition [35,36].
A major drawback of our study was the small sample size, which was probably due to the fact that the hospital is not a referral center for liver diseases. Nonetheless, the complete data gathered allowed for an in-depth study of the population and provided more data regarding the prognosis and treatment of cirrhosis in Brazil.
Conclusion
In conclusion, CLIF-SOFA score was superior to other liverspecific scores for predicting mortality in a cohort of a mixed public and private teaching hospital in Brazil, especially in values above 11. An increase in the use of these evidence-based scores may help define optimal diagnostic and therapeutic strategies for ACLF.
References
- Desmet VJ, Roskams T. Cirrhosis reversal: a duel between dogma and myth. J Hepatol. 2004; 40: 860-867.
- World Health Organization (WHO). Projections of mortality and burden of disease to 2030. 2018.
- Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003; 38: 258-266.
- Gustot T, Durand F, Lebrec D, Vincent JL, Moreau R. Severe sepsis in cirrhosis. Hepatology. 2009; 50: 2022-2033.
- Blei AT, Cordoba J. Practice Parameters Committee of the American College of Gastroenterology. Hepatic Encephalopathy. Am J Gastroenterol. 2001; 96: 1968-1976.
- Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010; 362: 823-832.
- Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010; 139: 1246-1256.
- Martin JA, Smith BL, Mathews TJ, Ventura SJ. Births and deaths: preliminary data for 1998. Natl Vital Stat Rep. 1999; 47: 1-45.
- Olson JC, Kamath PS. Acute-on-chronic liver failure: concept, natural history, and prognosis. Curr Opin Crit Care. 2011; 17: 165-169.
- Jalan R, Gines P, Olson JC, et al. Acute-on chronic liver failure. J Hepatol. 2012; 57: 1336-1348.
- Jalan R, Williams R. Acute-on-chronic liver failure: pathophysiological basis of therapeutic options. Blood Purif. 2002; 20: 252-261.
- Laleman W, Wilmer A, Evenepoel P, et al. Review article: non-biological liver support in liver failure. Aliment Pharmacol Ther. 2006; 23: 351-363.
- Roberts SE, Goldacre MJ, Yeates D. Trends in mortality after hospital admission for liver cirrhosis in an English population from 1968 to 1999. Gut. 2005; 54: 1615-1621.
- Das V, Boelle PY, Galbois A, et al. Cirrhotic patients in the medical intensive care unit: early prognosis and long-term survival. Crit Care Med. 2010; 38: 2108-2116.
- Warrillow SJ. Predictions and outcomes for the critically ill patient with cirrhosis: is it time to settle on the SOFA and let jaundiced views on outcome MELD away? Crit Care Med. 2010; 38: 2259-2260.
- Cholongitas E, Senzolo M, Patch D, et al. Risk factors, sequential organ failure assessment and model for end-stage liver disease scores for predicting short term mortality in cirrhotic patients admitted to intensive care unit. Aliment Pharmacol Ther. 2006; 23: 883-893.
- Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013; 144: 1426-1437.
- Soldera J, Balbinot SS, Balbinot RA, et al. Diagnostic and Therapeutic Approaches to Hepatocellular Carcinoma: Understanding the Barcelona Clínic Liver Cancer Protocol. Clin Med Insights Gastroenterol. 2016; 9: 67-71.
- Salerno F, Gerbes A, Ginès P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007; 56: 1310-1318.
- Tandon P, Garcia-Tsao G. Bacterial infections, sepsis, and multiorgan failure in cirrhosis. Semin Liver Dis. 2008; 28: 26-42.
- Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001; 33: 464-470.
- Kim WR, Biggins SW, Kremers WK, et al. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008; 359: 1018- 1026.
- Hernaez R, Solà E, Moreau R, et al. Acute-on-chronic liver failure: an update. Gut. 2017; 66: 541-553.
- Jalan R. Acute-on-chronic liver failure: from concept to a new syndrome. Curr Opin Crit Care. 2011; 17: 152
- Mookerjee RP. Acute-on-chronic liver failure: the liver and portal haemodynamics. Curr Opin Crit Care. 2011; 17: 170-176.
- Liu H, Lee SS. Acute-on-chronic liver failure: the heart and systemic hemodynamics. Curr Opin Crit Care. 2011; 17: 190-194.
- Cárdenas A, Ginès P. Acute-on-chronic liver failure: the kidneys. Curr Opin Crit Care. 2011; 17: 184-189.
- García-Martínez R, Cordoba J. Acute-on-chronic liver failure: the brain. Curr Opin Crit Care. 2011; 17: 177-183.
- Hassanein TI, Schade RR, Hepburn IS. Acute-on-chronic liver failure: extracorporeal liver assist devices. Curr Opin Crit Care. 2011; 17: 195-203.
- Dhiman RK, Agrawal S, Gupta T, et al. Chronic Liver Failure-Sequential Organ Failure Assessment is better than the Asia-Pacific Association for the Study of Liver criteria for defining acute-on-chronic liver failure and predicting outcome. World J Gastroenterol. 2014; 20: 14934-14941.
- Selva Rajoo A, Lim SG, Phyo WW, et al. Acute-on-chronic liver failure in a multi-ethnic Asian city: A comparison of patients identified by Asia-Pacific Association for the Study of the Liver and European Association for the Study of the Liver definitions. World J Hepatol. 2017; 9: 1133-1140.
- Silva PE, Fayad L, Lazzarotto C, et al. Single-centre validation of the EASLCLIF consortium definition of acute-on-chronic liver failure and CLIF-SOFA for prediction of mortality in cirrhosis. Liver Int. 2015; 35: 1516-1523.
- Hernaez R, Kramer JR, Liu Y, et al. Prevalence and Short-term Mortality of Acute-on-Chronic Liver Failure: a national cohort study from the USA. J Hepatol. 2018.
- Lee M, Lee JH, Oh S, Jang Y, Lee W, Lee HJ, et al. CLIF-SOFA scoring system accurately predicts short-term mortality in acutely decompensated patients with alcoholic cirrhosis: a retrospective analysis. Liver Int. 2015; 35: 46-57.
- Maipang K, Potranun P, Chainuvati S, Nimanong S, Chotiyaputta W, Tanwandee T, Charatcharoenwitthaya P. Validation of the prognostic models in acute-on-chronic liver failure precipitated by hepatic and extrahepatic insults. PLoS One. 2019; 14.
- Kim HY, Kim CW, Kim TY, Song DS, Sinn DH, Yoon EL, et al. Assessment of scoring systems for acute-on-chronic liver failure at predicting short-term mortality in patients with alcoholic hepatitis. World J Gastroenterol. 2016; 22: 9205-9213.