
Case Report
Austin J Gastroenterol. 2022; 9(1): 1119.
Pancreatic Solitary Fibrous Tumor as Result of Extracranial Metastatic Disease
López-Marte P¹* and Torres-Ortíz V²
¹University of Puerto Rico-School of Medicine, Division of Gastroenterology Research, San Juan, Puerto Rico
²Ponce Health Sciences University-School of Medicine, Division of Gastroenterology, Ponce, Puerto Rico
*Corresponding author: López-Marte P, Division of Gastroenterology Research, University of Puerto Rico-School of Medicine, PB-365067, San Juan, Puerto Rico
Received: March 02, 2022; Accepted: March 22, 2022; Published: March 29, 2022
Abstract
Solitary fibrous tumors and hemangiopericytomas (SFT/HPC) are an uncommon cause of pancreatic mass, and given its rarity data is limited. In this report, we present the case of a 61-year-old Female with history of SFT/HPC brain tumor, which was evaluated with abdominal imaging after presenting with an epigastric abdominal pain. A pancreatic mass was found and after endoscopic ultrasound- fine needle aspiration biopsy, she was found with extracranial metastatic disease. Our case adds clinical awareness and knowledge to this rare entity that needs to be considered when a pancreatic mass is found.
Keywords: Solitary fibrous tumor; Hemangiopericytomas; Pancreas; Endoscopic ultrasound; Extracranial metastatic disease
Introduction
Solitary fibrous tumors and hemangiopericytomas (SFT/HPC) are rare mesenchymal neoplasms that were thought to be different identities but during recent years it was found that both share the same etiology [1-5]. This type of tumor is caused by a fusion gene between NGFI-A Binding Protein 2 gene (NAB2) and signal transducer and activator of transcription 6 gene (STAT6) fusion gene that causes expression and accumulation of STAT6 protein in the cell nuclei [2,5]. STAT6 staining of tumor cell nuclei is known to be a very reliable marker and it has been used for definitive diagnosis [6,7]. SFT/HPC’s tumors are uncommon and represent less than 1% of all central nervous system (CNS) tumors [4]. Extracranial metastatic disease is rare [8-11]. In this report we describe a case of brain SFT/HPC with metastases to pancreas diagnosed using endoscopic ultrasound guided fine needle aspiration (EUS-FNA).
Case Presentation
A 61-year-old Female with prior history of brain SFT/HPC grade 3 with recurrence presents to the clinic complaining of epigastric abdominal pain. Family medical history was non-contributory. She denied any toxic habits. Physical examination was unrevealing. On further evaluation, her laboratory work-up including cell blood count, complete metabolic panel, pancreatic enzymes and CA 19-9 level were unremarkable. She did not have any history of pancreatic disorders. Abdominal enhanced CT scan showed a large heterogeneously enhancing mass in the pancreatic body with low density areas suggestive of necrosis measuring 7.8 cm x 6.7 cm as shown in Fig.2 on coronal (a) and axial views (b). For further assessment, the patient underwent EUS- guided FNA using a 22-gauge needle. Pathology description showed a paucicellular sample with patternless pattern of cells separated by thick collagenous material (Figure 1a). Immunohistochemistry (IHC) revealed a positive result for CD34 and STAT6 (Figure 1b and 1c). These results were consistent with previous brain histological and IHC results confirming metastatic disease to pancreas. After this diagnosis, an F-18 fluorodeoxyglucose (18-FDG) PET/CT scan demonstrated an area of 7.3 x 7.0 x 7.1cm of moderately hypermetabolic mass of the body of pancreas with SUV max of 7.91. There was no evidence of other organ involvement. Since the disease was limited to the pancreas, she underwent distal pancreatectomy. The pancreas neoplasm was described as pink-tan slightly lobulated mass covered by a capsule. The margins of resection were negative for neoplasia. The patient made an uneventful recovery and currently continues with imaging and clinical surveillance.
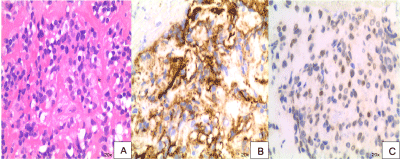
Figure 1: Hematoxylin and eosin stain of pancreatic mass showing paucicellular sample with patternless pattern of cells separated by thick collagenous material.
a) CD34 immunohistochemical stain; b) Nuclear expression of STAT6 on immunohistochemical stain; c) of cell block specimens.
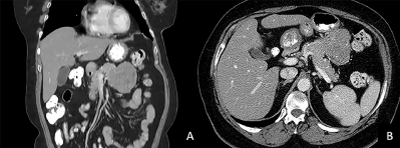
Figure 2: Image of abdominal enhanced CT scan showing a large well defined mass abutting from the body of pancreas in coronal (a) and axial views (b).
Discussion
Brain SFT/HPC’s are rare tumors of fibroblastic origin that were first described in 1996 by Carneiro et al. [1]. They can be found in any organ that contains mesenchymal cells. These neoplasms account for less than 1% of primary brain tumors and less than 30% of cases develop metastatic disease [12]. If metastatic disease occurs it is commonly found in the liver, pleura and bone [13]. Giordan et al. found that higher histological grade, degree of resection and recurrence is associated with metastases formation in intracranial SFT/HPC, and subsequent decreased survival [12]. Ghose et al., demonstrated in a systematic review of 523 patients that the mean time after first diagnosis of brain SFT/HPC to develop metastasis was around 8 years [4]. Comparing these data, our patient had multiple brain recurrences and developed metastatic disease 17 years after being first diagnosed. This could be related due to incomplete resection or the presence of positive margins when she underwent brain tumor mass excision, which remains unknown. Last update of CNS tumors classification by World Health Organization (WHO) included grading as a malignancy scale based on histologic features with tumors showing 5 or more mitoses per high- power to be graded as III and to have the potential for recurrence and to long term risk for metastasis [8]. It has been proposed that recurrence, regardless of tumor primary site, could also be predicted by the presence of Ki- 67 >5% [3]. Only a few cases of SFT/HPCs tumors in pancreas have been reported, with abdominal pain and jaundice being the most common clinical manifestation with the majority of patients being asymptomatic [14]. The majority of these tumors have an indolent course, unpredictable behavior, and the lack of a proven, reliable and easy to reproduce predictive model make it difficult for physicians to identify patients at risk for metastases. More research about the pathogenesis of this condition is needed in order to establish an adequate surveillance and medical management.
References
- Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH. Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol. 1996; 106: 217-224.
- DeVito N, Henderson E, Han G, et al. Clinical Characteristics and Outcomes for Solitary Fibrous Tumor (SFT): A Single Center Experience. PLoS One. 2015; 10: e0140362.
- Yamamoto Y, Hayashi Y, Murakami I. Recurrence of Solitary Fibrous Tumor/ Hemangiopericytoma Could Be Predicted by Ki-67 Regardless of Its Origin. Acta Med Okayama. 2020; 74: 335-343.
- Ghose A, Guha G, Kundu R, Tew J, Chaudhary R. CNS Hemangiopericytoma: A Systematic Review of 523 Patients. Am J Clin Oncol. 2017; 40: 223-227.
- Yamashita D, Suehiro S, Kohno S, et al. Intracranial anaplastic solitary fibrous tumor/hemangiopericytoma: immunohistochemical markers for definitive diagnosis. Neurosurg Rev. 2021; 44: 1591-1600.
- Berghoff AS, Kresl P, Bienkowski M, et al. Validation of nuclear STAT6 immunostaining as a diagnostic marker of meningeal solitary fibrous tumor (SFT)/hemangiopericytoma. Clin Neuropathol. 2017; 36: 56-59.
- Macagno N, Figarella-Branger D, Mokthari K, et al. Differential Diagnosis of Meningeal SFT-HPC and Meningioma: Which Immunohistochemical Markers Should Be Used? Am J Surg Pathol. 2016; 40: 270-278.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131: 803-820.
- Yamashita H, Fujino Y, Ohara T, et al. A rare case of metastatic solitary fibrous tumor of the pancreas manifesting as a cystic neoplasm: a case report. Surg Case Rep. 2019; 5: 142.
- Varghese L, Ngae MY, Wilson AP, Crowder CD, Gulbahce HE, Pambuccian SE. Diagnosis of metastatic pancreatic mesenchymal tumors by endoscopic ultrasound-guided fine-needle aspiration. Diagn Cytopathol. 2009; 37: 792- 802.
- Osuga T, Hayashi T, Ishiwatari H, et al. Pancreatic metastasis from a solitary fibrous tumor of the central nervous system. JOP. 2014; 15: 58-62.
- Giordan E, Marton E, Wennberg AM, Guerriero A, Canova G. A review of solitary fibrous tumor/hemangiopericytoma tumor and a comparison of risk factors for recurrence, metastases, and death among patients with spinal and intracranial tumors. Neurosurg Rev. 2021; 44: 1299-1312.
- Lavacchi D, Antonuzzo L, Briganti V, et al. Metastatic intracranial solitary fibrous tumors/hemangiopericytomas: description of two cases with radically different behaviors and review of the literature. Anticancer Drugs. 2020; 31: 646-651.
- Li J, Li J, Xiong Y, et al. Atypical/malignant solitary fibrous tumor of the pancreas with spleen vein invasion: Case report and literature review. Medicine (Baltimore). 2020; 99: e19783.