Review Article
Austin J Genet Genomic Res. 2014;1(1): 6.
Contribution of Genetics in the Recent Human Evolution Study: Knowledge, Problems and Future Prospects
Hassen Chaabani*
Laboratory of Human Genetics and Anthropology, University of Monastir, Tunisia
*Corresponding author: Hassen Chaabani, Laboratory of Human Genetics and Anthropology, University of Monastir, 5000 Monastir, Tunisia
Received: June 20, 2014; Accepted: July 14, 2014; Published: July 16, 2014
Abstract
The study of human evolution involves several scientific disciplines particularly paleontology, archeology and genetics. The research in the latter has provided new insights into the evolutionary relationships of human populations leading to an improved understanding of their origin and their migration across the globe. In fact, mutations on genes and non-coding DNA sequences occurred since our deep evolutionary past represent precious traces the analyses of which permit to refer the past from present. The research development of human evolutionary genetic studies has been passed through two principal stages. In this paper I present briefly the most important general conclusions obtained during these two stages. Besides, I present and discuss emerged problems concerning particularly the persistence of some problematic considerations and confusions and vagueness related to some concepts. I believe that it is time to uproot all problematic considerations and resolve all other problems that have curbed the progression of research in this topic and to move on new more objective and more empirical research tracks.
Keywords: Human evolutionary genetic studies; Protein markers; DNA markers; Research problems; Modern man origin; Questionable considerations; Research approaches
Introduction
The subject of the origin of humans and their evolutionary history had been theoretically somewhat presented within the notion of the general biological evolution idea in many ancient writings of some Islamic Renaissance scholars in the wider Middle East such those of the Iraqi thinker and writer Amr ibn Bahr Al Jahis (800-868) in his famous work presented in his book "Book of Animals", those of Ibn al-Haytham (1000-1038) who argued for evolutionism and Ibn Miskawayh during the same period (1000-1030) discussed ideas on evolution. Then those of Ibn Kaldoun (1332 - 1406) who presented this subject with relatively more accuracy in abridged paragraphs in his book "Muqaddimah" [1]. Later, in 1859 Charles Darwin presented a more detailed concept on the biological evolution in writing "Origin of Species" and on the human origin in his book entitled "The Descent of Man and Selection in Relation to Sex" (1871) in which he argued that all of the known evidence was consistent with humans having evolved from a common ancestor shared with apes. He speculated that Africa was their place of origin and that human ancestors had gradually taken on their current form since then.
The first practical preliminary research works related to this subject has been started from the beginning of the 19th century. They concern the study of the anatomy of our ancestors through the investigation of discovered human fossils or the study of the biological diversity of contemporary human populations. The latter type of study has been developed with the development of the field of genetics passing through two principal stages. The first stage, mainly marked by the discovery and use of protein markers (classic markers), has been developed within the branch of "Genetics of Human Populations". The second, started by the emergence of molecular biology technology and marked by the determination and use of DNA markers, has been developed mainly within a more specific new branch designated "Genetic Anthropology or Molecular Anthropology". In this paper I present briefly the most important general conclusions obtained during these stages. Besides, I present and discuss emerged problems and I give resolutions and perspectives that could lead to new research approaches more adequate for this subject.
Brief Overview of the most Important Literature Data
The study of the human biological diversity was firstly based on the unsuitable use of macroscopic (phenotypic) characters such as the skin color and then on the correct and fruitful use of microscopic characters represented firstly by the protein markers. The ABO blood group antigens are the first protein markers used to characterize human populations. In fact, since 1919, several populations were studied according to these antigens. The analysis of results shows that the B allele frequencies are regularly quite higher in Asians than in Europeans and only exceptional populations are characterized by the absence of one or two of the three ABO blood groups alleles, like the South American Indians only have the O allele.
Later on, other blood group systems were discovered; for example the Diego blood group antigen that firstly was found in Diego Indians living in Venezuela and, therefore, considered as typical to these Indians. But its discovery in North American Indians and in the East Asian populations suggested an Asian origin of the American Indians. I can quote also the blood group antigens of the Duffy system, which has three alleles of importance Fya, Fyb and a silent allele Fy that is almost entirely restricted to Africans [2].
Since then, several protein systems have been described and progressively studied in depth [3,4]. I can quote the case of Pi system and that of the Haemoglobin. The latter presents, beside the common Haemoglobin A (HbA) variant, more than 300 rare variants. Some of them present a limited population of origin such as the case of HbE variant typical to the Cambodian population. The Pi system presents more that 30 alpha1-antitrypsin variants. Excepted the subtypes of the common variant M, all variants are rare such as the Pclifton variant characteristic of the sub-Saharan Africans [5]. As these examples of blood group and proteins systems show, certain allelic variants occurred in single population could be served as unique population markers, but their presence in low frequencies limits their anthropological usefulness.
On the other hand, three highly polymorphic systems, Rhesus, GM and HLA, were discovered and used in the study of genetic differentiation of human populations [6]. The Rhesus system presents eight major haplotypes at closely linked loci or a complex locus on chromosome 1. For this system the r haplotype is common in Europeans and North Africans but absent or scarce in Orientals, Oceanians and American Indians; while the high frequency of R° characterizes the sub-Saharan Africans [7,8,9]. Besides, analyses of the variation of Rh haplotype frequencies among worldwide populations provided an accurate anthropological picture on the human evolutionary relationships [9]. Concerning the immunoglobulin GM system represents the polymorphism of γ1, γ2 and γ3 heavy chain constant regions of human immunoglobulin. These polymorphisms represent a matter of allotypic determinants designated GM allotypes that, considered as neutral or quasi neutral markers, are encoded by closely linked alleles on chromosome 14. These alleles are co-dominantly inherited in specific combinations or haplotypes. The analyses of genetic distances corresponding to GM haplotypes frequencies give a clear network of genetic relationships of world populations in a general correspondence with geography coupled to historical patterns of gene flow and genetic drift influence [8,10]. In addition the phylogeny of these haplotypes themselves could contribute to reconstruct the principal stages of the human evolutionary history [11]. On the other hand although the HLA system is the most polymorphic its use in anthropological studies is relatively limited because the nature of its polymorphism and molecular sequence variation in its genes support the idea that these genes are under natural selection [12,13]. However, some studies on several populations have been carried out such as those which, limited to 57 HLA-A, B, C antigens, showed that HLA alleles found in European populations are generally the same observed in Africans with difference in frequencies, while some HLA alleles are restricted to Asian populations [14,15].
Since 1980th, developments in DNA and computer technologies have revolutionized the study of recent human evolution. Several types of DNA polymorphism have been identified and used in the coding sequences [16,17] and non-coding parts of the human genome such as the single nucleotide polymorphisms (SNPs) [18], the repeat length polymorphisms [19-21] and the uni-parental, mitochondrial [22,23] and Y chromosome DNA polymorphisms considered as the most useful for studying historical population movements [24-26].
Rigorous worldwide populations' studies were carried out using especially high number of DNA markers. They showed that 85% to 95% of human diversity is due to differences between individuals of the same population, whereas differences among continental groups account for 3% to 10% of the overall genetic variance [27]. This surprisingly small amount of genetic variation so noted throughout all present-day human populations is considered among the most precious scientific conclusions that represent a crowning achievement of the 20th century. Besides, innumerable studies were carried out using one of the different types of DNA markers at a micro and macro-geographic scales providing new insights into historical and demographical questions such as the use of the Alu insertions known by their potential usefulness as ancestry informative markers [28-32]. DNA markers were also analyzed in attempts to determine the place and / or the time of modern man emergence [33,34].
Emerged Problems, Discussion and Prospects
Early problem
The first emerged problem is the classification of human populations in races and then in major human groups. In fact some classic anthropologists had classed races inside humans by placing unreal limits in the continued variations of the morphological characters particularly the skin color. But this consideration was strongly rejected after the discovery and analyses of protein and DNA markers. For example, if we consider the skin color as racial character, the black populations of sub-Saharan Africa and South Asia must be classed within the same race. This is inconsistent with the distribution of protein and DNA markers that show a significant difference between these two geographically distant populations [35]. In addition, the pattern of small amount of genetic variation noted throughout all present-day human populations is strongly against any racial classification. In fact, the black color would have been only a genetic adaptation to the tropical climate and recent genetic studies indicate that skin color may change radically over as few as 100 generations, or about 2,500 years, given the influence of the environment [36]. Such correlation existing between this morphological character and climate could lead to a convergent evolution of population living in similar climates enough to obscure the phylogenetic trees of human population.
From the 1980th the majority of genetic-anthropologists have avoided using the term "race" for speaking about human population group designations, namely the latter have indirectly replaced or masked those of human races. In fact they have attempted to arrange present populations in three major human groups appointed: Caucasoid, Mongoloid and Negroid. The origin of the Caucasoid designation could be "Caucasia" geographic region in the South East of Europe. This does not show a clear correspondence with its present vague sense, which appoints all "white" populations, either only Europeans or both Europeans and other populations as those of North Africa and Middle East. The Negroid term, stemming probably from "Negro" and appointing in the beginning all "black" populations, is restricted now to "black" Africans because of their genetic profile significantly different from all other human populations including "black" populations from other countries. The Mongoloid term, appointed populations from Mongolia was often used to designate East Asian populations with or without Oceania populations and Amerindians.
I believe that at present it is inconceivable to keep on with these designations because in addition of their imprecise and inappropriate sense, they cannot include all world populations particularly those living in intermediate geographical regions and assure the continuity of the genetic variation between populations of different continents. In other words it is not possible to class present human populations in three major groups because they represent a global complex network of genetic relationships, which reflects mainly their unique origin and their migration and isolation history since the recent emergence of modern man [11,34]. Thus in light of all these scientific considerations, more precise and adequate designations of human populations referring to their countries have been used such as "Tunisian population" or to a larger geographic area to which belonged their countries ("North African population" or "South Mediterranean population"). At present, although the majority of anthropologists follow these correct designations unfortunately some of them from time to time continue to use, in more and less concealed state, the racial terminology or major human group designations. I believe that it is odd to continue to talk about these terminology and designations and even if some of them want to design by "race" a human group having distinct cultural features, they must use the term "ethnic group" and not that of "race".
Principal current problems
The current problems of the studies on recent human evolution concern mainly the research of date and place of the modern human emergence. The first radical problem that I consider as the cause of the majority of confusions, vagueness, debates and controversy concerns the determination and definition of modern man himself "Homo sapiens sapiens". The second concerns distortions, confusions and vagueness on the theory of recent and unique origin of modern man.
Concerning the first problem, classic paleoanthropologists have used the analyses of general anatomical futures and particularly the discrete cranial traits (DCT) for determining and defining modern humans and therefore for differentiating Homo erectus fossils from those of modern humans. But recent rigorous studies [37-39] have shown that the post cranial morphology of Homo erectus, although more robust, falls within the range of that of Homo sapiens sapiens; while the use of DCT leads to questionable identifications [40,41] in disagreement with corresponding ancient DNA data [42,43]. Hence the anatomical criterion is evidently of limited utility in identifying the true modern humans' fossils and in reconstructing their past. Similar general conclusions are already noted in several recent rigorous studies [39,44-46]. As prospect we must look for another adequate criterion that permits a more adequate definition and therefore a more valid identification of modern humans such as my proposition to consider the brain complexity responsible for the superior potential cognitive abilities as the principal constant criterion that marks strongly the definition of modern man since his emergence [34].
Although the evident limited utility of anatomical criterion for identifying modern humans so revealed by recent rigorous data, the date of modern man emergence estimated from anatomical future analyses to about 100,000- 120,000 years ago [47] is yet popular. This represents a good example that shows how evident rigorous recent data are somewhat neglected or concealed beside the hegemony of the current of a classic questionable consideration. Besides, this date corresponding to the age of earliest fossils supposed as anatomically modern is different from that found by Cann et al. [33] who, on the basis of mtDNA analyses, estimated it to about 200,000 years ago with error interval ranges from simple to double. This surprising large interval, among other things, reflects the fact that they have used problematic methods. In fact, these methods are sophisticated, theoretical and not sufficiently stable, and particularly include problematic assumptions such as that of the mutation rate of the human mtDNA evolution [48-51]. Later, different dates have been published such as that estimated to 137,000 ± 15,000 years ago [52] from autosomal markers analysis or to 142,000 years ago from the Y chromosome markers analysis [25]. In any case, whatever the degree of the validity of different methods applied on different genetic data, the obtained dates do not correspond necessary to that of modern man emergence but they could extend back to any point in the Homo evolutionary history. Moreover these dates do not agree with conclusions deduced from rigorous genetic analyses on different DNA sequences such as (1) the estimation average age of Alu insertions divergence of between 30,000 and 55,000 years that provides further support for a recent worldwide human replacement [53], (2) the individual whole genome sequences analysis that shows considerable genetic exchanges may still have occurred until 20-40 kyr ago [54], (3) the genomic surveys in humans identify a large amount of recent positive selection [55] likely occurred in the last 10,000 - 40,000 years [56,57]. In fact, the positive selection promotes the emergence of new phenotypes and can leave a set of telltale signatures in the genes under its influence, such as the rapid divergence of functional sites between species and the depression of polymorphism within species [58,59]. In addition, authors of these works showed a category of genes for which positive selection appears to have operated more intensely in the lineage leading to humans than in other lineages. These genes, often associated with behavior and brain development, are particularly relevant to understanding the evolution of biological traits as advanced cognitive abilities that distinguish our species and sub-species sapiens sapiens [60]. Hence, all these conclusions and explanations are in favor of a real recent date of modern man emergence such as my proposed dates of 45,000 and 20,000 years ago for the emergence of our species and subspecies respectively [34].
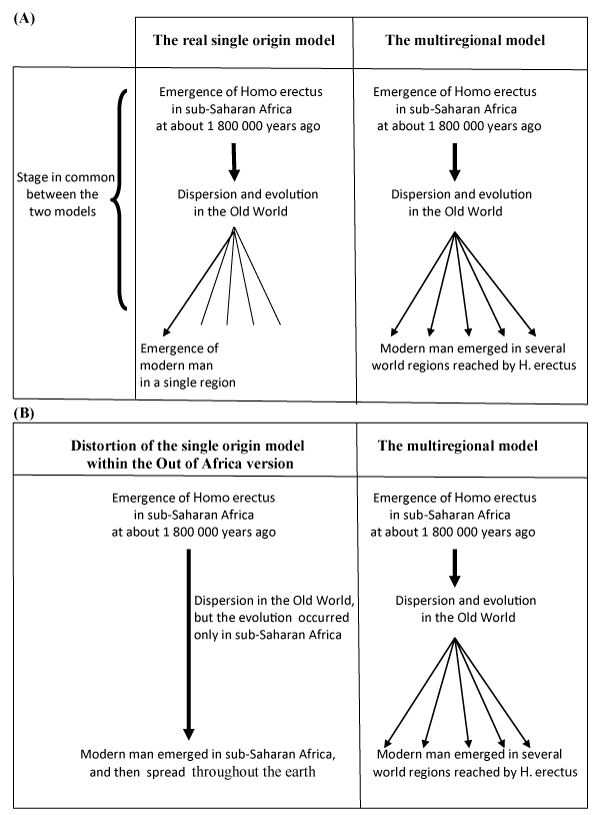
The second problem is linked up with the model of the single origin of human populations. Accepting the fact that our evolution was started from sub-Saharan Africa, this model suggests that all current human populations descend from a single ancestral population of modern humans who, spread throughout the earth, having completely replaced the preceding archaic populations without interbreeding; while the model of the multiregional origin argues that the early Homo peoples migrated out of Africa in different parts of the world where they had continued independently their evolution in modern humans. As show these descriptions, oversimplified in Figure 1 (A), both models would agree with the fact that the first Homo peoples appeared in sub-Saharan Africa then spread in different world continent, where they had continued their evolution. But they are evidently opposed only for the last period of the human evolutionary history concerning the modern man emergence, which occurred in a unique place without interbreeding with preceding archaic populations for the model of single origin and in several world regions for the model of multiregional origin. The surprisingly small amount of genetic variation throughout all present-day human populations [27] and linguistic data [61,34] have supported strongly the model of single origin and showed that our origin is not only unique but also recent and consequently this model become a general theory: the theory of unique and recent origin of modern humans.
Figure 1 : A. Diagrammatic representation of the two usual human evolution models. B. Distortion of the single origin model linked up with the Out of Africa version.
Since the publication of Cann et al. in 1987 [33] two principal confusions occurred on this theory. First, several authors have confused this well-accepted general theory with that of the so-called 'Out of Africa'. In fact in the latter, although the principle of the general theory has been adopted, the date and the place of modern man emergence, that still represent a controversy, have been proposed. Hence the 'Out of Africa' could be considered as one of possible several versions of the theory and not the theory itself [34]. Second some anthropologists who align on the 'Out of Africa' version imply that all evolution and divergences of human species and subspecies were happened only in sub-Saharan Africa. But this consideration could not be accepted: in other words how the earliest Homo peoples emerged in sub-Saharan Africa and spread in several regions of the world but they had evolved only in sub-Saharan Africa? In fact this consideration get away from the well accepted theory according to which only the divergence of the modern man were accomplished in a single place while that of his predecessors, such as archaic Homo sapiens or some eventual sub-species of Homo erectus, could be diverged in different regions of the Old World [34]. Who is concerned by this second confusion seems to be inclined to make this theory completely different from the model of multiregional origin Figure 1 (B) within a general human tendency to develop two diametrically opposing visions, then the alignment on one of them. The problem is that the alignment often occurs without presenting new convincing arguments as it was done by several authors towards the out of Africa hypothesis.
Conclusion
In conclusion it is interesting to note that at present we are found in front of a great deal of data stored up from research works related to the recent human evolution subject. But unfortunately these intense accumulating data particularly during the two last decades were not leaded to an evident progress in this subject although some rigorous data with new conclusions have been published. In fact these new conclusions are often neglected or concealed beside the hegemony of some classic and / or popular considerations although these latter appear more and more problematic and unconvincing. If this situation will continue the present braking of the research progression in this subject will continue. I believe that it is time to uproot all these problematic considerations, confusions and vagueness and then to move on new more objective and more empirical research tracks. A model of such research approach was followed in the development of a new version of the theory of unique and recent origin of modern humans, designated "Recent out of Yemen" thesis [34]. The expansion of such new research context could make the field of genetic anthropology a science of future.
References
- Chaabani H. Insights on the history of Anthropology: its emergence in the wider Middle East before it existed as a discipline. Int J Mod Anthrop. 2012; 5: 80-87.
- Mourant AE, Kopec AC. In: The Distribution of the Human Blood Groups and Other Pohymorphisms (Fourquet R, Personal Communication). Domaniewska-Sobczak K, Editor. London: Oxford University Press. 1976.
- Giblett ER. Genetic Markers in Human Blood. Wiley & Sons, Limited, John, USA. 1969.
- Harris H. The principles of human biochemical genetics. 3rd revised edition. Elsevier/North-Holland Biomedical Press, Amsterdam. 1980; 554.
- Chaâbani H, Cox DW. Genetic characterization and origin of Tunisian Berbers. Hum Hered. 1988; 38: 308-316.
- Sanchez-Mazaz A, Pellegrini B. Rhesus, GM and HLA and modern humans' history. Bull et Mém de la Soc d' Anthrop de Paris. 1990; 2: 57-76.
- Langaney A. Diversité et histoire humaine. In: Population, 34e année, n6. 1979; 985-1006.
- #Chaabani H, Sanchez-Mazas A, Sallami SF. Genetic differentiation of Yemeni people according to rhesus and Gm polymorphisms. Ann Genet. 2000; 43: 155-162.
- Ben Halima A, Bahri R, Esteban E, Moral P, Chaabani H. Variation of rhesus haplotype frequencies in North Africans and in worldwide population analyses. Int J Hum Genet. 2014, [in process].
- Matsumoto H. Characteristics of Mongoloid and neighboring populations based on the genetic markers of human immunoglobulins. Hum Genet. 1988; 80: 207-218.
- Chaabani H. GM polymorphism and the evolutionary history of modern humans. Ann Genet. 2002; 45: 197-206.
- Meyer D, Thomson G. How selection shapes variation of the human major histocompatibility complex: a review. Ann Hum Genet. 2001; 65: 1-26.
- Hirayasu K, Ohashi J, Tanaka H, Kashiwase K, Ogawa A. The nature of polymorphism and molecular sequence variation in the genes of the human major histocompatibility complex (MHC) provides strong support for the idea that these genes are under selection. Am J Hum Genet. 2008; 82: 1075-1083.
- Degos L, Jacqcquard A, Landre MF, Salmon E, Valat MT. Study of distances between populations from the variation of HL-A gene frequencies. Histocomatibility Testing, Munksgaard, Copenhague. 1972; 739-744.
- Degos A, B, C, D de HLA Editions Masson, Paris, 1988.
- Chaabani H, Bech-Hansen NT, Cox DW. Restriction fragment length polymorphisms associated with immunoglobulin heavy chain gamma genes in Tunisians. Hum Genet. 1986; 73: 110-113.
- Wainscoat JS, Hill AVS, Boyce AL, Flint J, Hernandez M, Thein SL, et al. Evolutionary relationships of human populations from an analysis of nuclear DNA polymorphisms. Nature. 1986; 319: 491-493.
- Xing J, Watkins WS, Witherspoon DJ, Zhang Y, Guthery SL, Thara R, et al. Fine-scaled human genetic structure revealed by SNP microarrays. Genome Res. 2009; 19: 815-825.
- Batzer MA, Deininger PL, Hellmann-Blumberg U, Jurka J, Labuda D, Rubin CM. Standardized nomenclature for Alu repeats. J Mol Evol. 1996; 42: 3-6.
- Stoneking M, Fontius JJ, Clifford SL, Soodyall H, Arcot SS, Saha N, et al. Alu insertion polymorphisms and human evolution: evidence for a larger population size in Africa. Genome Res. 1997; 7: 1061-1071.
- Terreros MC, Alfonso-Sánchez MA, Novick GE, Luis JR, Lacau H, Lowery RK, et al. Insights on human evolution: an analysis of Alu insertion polymorphisms. J Hum Genet. 2009; 54: 603-611.
- Denaro M, Blanc H, Johnson MJ, Chen KH, Wilmsen E, Cavalli-Sforza LL, et al. Ethnic variation in Hpa 1 endonuclease cleavage patterns of human mitochondrial DNA. Proc Natl Acad Sci U S A. 1981; 78: 5768-5772.
- Cerný V, Mulligan CJ, Fernandes V, Silva NM, Alshamali F, Non A, et al. Internal diversification of mitochondrial haplogroup R0a reveals post-last glacial maximum demographic expansions in South Arabia. Mol Biol Evol. 2011; 28: 71-78.
- Hammer MF. A recent common ancestry for human Y chromosomes. Nature. 1995; 378: 376-378.
- Cruciani F, Trombetta B, Massaia A, Destro-Bisol G, Sellitto D, Scozzari R, et al. A revised root for the human Y chromosomal phylogenetic tree: the origin of patrilineal diversity in Africa. Am J Hum Genet. 2011; 88: 814-818.
- Cadenas AM, Zhivotovsky LA, Cavalli-Sforza LL, Underhill PA, Herrera RJ. Y-chromosome diversity characterizes the Gulf of Oman. Eur J Hum Genet. 2008; 16: 374-386.
- Barbujani G, Magagni A, Minch E, Cavalli-Sforza LL. An apportionment of human DNA diversity. Proc Natl Acad Sci U S A. 1997; 94: 4516-4519.
- El Moncer W, Esteban E, Bahri R, Gayá-Vidal M, Carreras-Torres R, Athanasiadis G, et al. Mixed origin of the current Tunisian population from the analysis of Alu and Alu/STR compound systems. J Hum Genet. 2010; 55: 827-833.
- Bahri R, El Moncer W, Al-Batayneh K, Sadiq M, Esteban E, Moral P, et al. Genetic differentiation and origin of the Jordanian population: an analysis of Alu insertion polymorphisms. Genet Test Mol Biomarkers. 2012; 16: 324-329.
- Bahri R, Ben Halima A, Ayadi I, Esteban E, Alfadhli S, Rebai A, et al. Genetic position of Bahrain natives among wider Middle East populations according to Alu insertion polymorphisms. Annals of Human Biology. 2013; 40: 35-40
- Halima AB, Bahri R, Esteban E, Aribia MH, Moral P, Chaabani H, et al. Ethnic composition and genetic differentiation of the Libyan population: insights on Alu polymorphisms. Ann Hum Biol. 2014; 41: 229-237.
- Salem AH, Bahri R, Jarjanazi H, Chaabani H. Geographical and social influences on genetic diversity within the Egyptian population: analyses of Alu insertion polymorphisms. Ann Hum Biol. 2014; 41: 61-66.
- Cann RL, Stoneking M, Wilson AC. Mitochondrial DNA and human evolution. Nature. 1987; 325: 31-36.
- Chaabani H. Recent out of Yemen: new version of the theory of unique and recent origin of modern man. Int J Mod Anthrop. 2014; 7: 13-41
- Nei M, Roychoudhury AK. Evolutionary relationships of human populations on a global scale. Mol Biol Evol. 1993; 10: 927-943.
- Jablonski NG. Living color: The biological and social meaning of skin color. University of California Press, 1ST edition. 2012.
- Wood B, Collard M. The human genus. Science. 1999; 284: 65-71.
- Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004; 432: 345-352.
- Tattersall I, Schwartz JH. The Morphological Distinctiveness of Homo sapiens and Its Recognition in the Fossil Record: Clarifying the Problem. Evolutionary Anthropology. 2008; 17: 49-54.
- Gish DT. Evolution: the fossils still say no! An updated version of Gish 1985. El Cajon, CA: Institute for Creation Research. 1995.
- Caspari P, Wolpoff H. The process of modern human origins. In: The origin of modern humans 'Biology reconsidered'. Smith FH, Ahern JCM, editors. New Jersey: John Wiley and Sons. 2013.
- Adcock GJ, Dennis ES, Easteal S, Huttley GA, Jermiin LS, Peacock WJ, et al. Mitochondrial DNA sequences in ancient Australians: Implications for modern human origins. Proc Natl Acad Sci U S A. 2001; 98: 537-542.
- Relethford JH. Ancient DNA and the origin of modern humans. Proc Natl Acad Sci U S A. 2001; 98: 390-391.
- Wolpoff MH. Describing anatomically modern Homo sapiens: a distinction without a de? nable diVerence. In: Fossil Man. New Facts, New Ideas. Papers in Honor of Jan Jelínek's Life Anniversary. Novotny VV, Mizerová A, Editors. Anthropos (Brno). 1986; 23: 41-53.
- Brown P. Osteological de? nitions of ''anatomically modern'' Homo sapiens: a test using modern and terminal Pleistocene Homo sapiens. In: Is Our Future Limited by Our Past? Proceedings of the Third Conference of the Australasian Society of Human Biology. Freedman L, Editor. Centre for Human Biology. University of Western Australia, Nedlands. 1990; 51-74.
- Kidder JH, Jantz RL, Smith FH. De? ning modern humans: a multivariate approach. In: Continuity or Replacement? Controversies in Homo sapiens Evolution. Bräuer G, Smith FH, Editors. Rotterdam: Balkema. 1992; 157-177.
- Valladas H, Reyes JL, Joron JL, Valladas G, Bar Yosef O, Vandermersch B. Thermoluminescence dating of Mousterian Proto-Cro Magnon remains from Israel and the origin of modern man. Nature. 1988; 331: 614-616.
- Excoffier L, Langaney A. Origin and differentiation of human mitochondrial DNA. Am J Hum Genet. 1989; 44: 73-85.
- Vigilant L, Stoneking M, Harpending H, Hawkes K, Wilson AC. African populations and the evolution of human mitochondrial DNA. Science. 1991; 253: 1503-1507.
- Maddison DR. "African origin of human mitochondrial DNA reexamined". Syst Zool. 1991; 40: 355-363.
- Klyosov AA. Reconsideration of the "Out of Africa" Concept as Not Having Enough Proof. Advances in Anthropology. 2014; 4: 18-37.
- Stoneking M, Fontius JJ, Clifford SL, Soodyall H, Arcot SS, Saha N, et al. Alu insertion polymorphisms and human evolution: evidence for a larger population size in Africa. Genome Res. 1997; 7: 1061-1071.
- Knight A, Batzer MA, Stoneking M, Tiwari HK, Scheer WD, Herrera RJ, et al. DNA sequences of Alu elements indicate a recent replacement of the human autosomal genetic complement. Proc Natl Acad Sci U S A. 1996; 93: 4360-4364.
- Li H, Durbin R. Inference of human population history from individual whole-genome sequences. Nature. 2011; 475: 493-496.
- Voight BF, Kudaravalli S, Wen X, Pritchard JK. A map of recent positive selection in the human genome. PLoS Biol. 2006; 4: e72.
- Wang ET, Kodama G, Baldi P, Moyzis RK. Global landscape of recent inferred Darwinian selection for Homo sapiens. Proc Natl Acad Sci U S A. 2006; 103: 135-140.
- Hawks J, Wang ET, Cochran GM, Harpending HC, Moyzis RK. Recent acceleration of human adaptive evolution. Proc Natl Acad Sci U S A. 2007; 104: 20753-20758.
- Kreitman M. Methods to detect selection in populations with applications to the human. Annu Rev Genomics Hum Genet. 2000; 1: 539-559.
- Bamshad M, Wooding SP. Signatures of natural selection in the human genome. Nat Rev Genet. 2003; 4: 99-111.
- Vallender EJ, Lahn BT. Positive selection on the human genome. Hum Mol Genet. 2004; 13 Spec No 2: R245-254.
- Shevoshkin V. Reconstructing Languages and Cultures. Shevoshkin V, editor. Bochum: Brockmeyer. 1989.