
Case Presentation
Austin J Genet Genomic Res. 2015;2(1): 1009.
Co-Existence of Two Abnormal Clones: Diploidy with a Unique Inverted 5q Deletion Not Involving EGR1 and Near-Tetraploidy with Monosomy 7 in Biphenotypic Leukemia Arising from Myelodysplastic Syndrome
Ramlal R¹, Lionberger JM¹, Robbins KJ² and Batanian JR³*
¹Department of Internal Medicine, Saint Louis University Medical Center, USA
²Department of Pathology, Saint Louis University Medical Center, USA
³Department of Pediatrics Molecular Cytogenetics, SSM Cardinal Glennon Children's Medical Center, USA
*Corresponding author: Batanian JR, Department of Pediatrics Molecular Cytogenetics, SSM Cardinal Glennon Children's Medical Center, 1465 S Grand Blvd, St. Louis, MO 63104, USA
Received: March 02, 2015; Accepted: April 02, 2015; Published: April 06, 2015
Abstract
Biphenotypic Acute Leukemias (BAL) account for less than 5% of all cases of acute leukemias. Cytogenetic abnormalities are very common in BAL occurring in 59-91% of cases. These leukemias are generally associated with a poor prognosis with no clear consensus existing on their optimal management. We report a case of a biphenotypic acute leukemia arising in a patient with a prior history of Myelodyplastic Syndrome (MDS) expressing two cytogenetically distinct clones: a diploid clone with a unique 5q inversion/ deletion not involving EGR1 and a second clone of cells with a near -tetraploidy having monosomy 7 as the main numerical abnormality. The patient is currently alive and remains in remission seventeen months following diagnosis of his BAL after undergoing induction chemotherapy with idarubicin, cytarabine and cladribine and a consolidative double cord blood transplant.
Keywords: Inversion deletion 5q not involving EGR1; Monosomy 7 masked by near-tetraploidy; Biphenotypic phenotype
Abbreviations
a-CGH: Array-Comparative Genome (a-CGH) Hybridization; AML: Acute Myeloid Leukemia; ALL: Acute Lymphoblastic Leukemia; BAL: Biphenotypic Acute Leukemia; CEBPA: CCAAT/ Enhancer-Binding Protein, Alpha; CR1: First Complete Remission; DFS: Disease Free Survival; EGIL: European Group for the Immunological Classification of Acute Leukemias; EGR1: Early Growth Response Protein 1; FLT3-ITD: FMS-like Tyrosine Kinase 3 – Internal Tandem Duplications; FISH: Fluorescence In Situ Hybridization; MDS: Myelodysplastic syndrome; MPAL: Mixed Phenotype Acute Leukemia; NPM1: Nucleophosmin 1
Case Presentation
A 66 year old man with a history of 5(q-) MDS diagnosed in 2004 presented with profound fatigue, chest pains and anemia. He had received treatment with Lenalidomide on and off over the past eight years as well as erythropoietin stimulating agents. He presented to St Louis University Hospital Cancer Center for evaluation of pancytopenia in August of 2013. His complete blood count at his initial clinic visit showed a hemoglobin level of 8.2 g/dl, a platelet count of 90,000/μL, and a white blood cell count of 1800/μL with 29% neutrophils, 61.5% lymphocytes, with no immature forms reported.
Pathology
A bone marrow biopsy was performed which revealed an overall cellularity of 50% with 53.5% blast, 9.5% myeloid precursors, 33.5% erythroid progenitors, 1.5% lymphocytes, 1% eosinophils and 1% monocytes. The blasts were small to intermediate in size, with cellular border irregularity, prominent nucleoli and moderate amount of agranular variable basophilic cytoplasm with occasional vacuoles and cytoplasmic blebs. The erythroid maturation was profoundly dyserythropoietic. The megakaryocytes were decreased in number and abnormal in morphology with the majority of them small and hypolobated in appearance (Figure 1A, 1B and 1C).

Figure 1a: The decalcified bone marrow trephine core biopsy demonstrates
variable cellularity, with an overall average cellularity of 50% (hematoxylin
and eosin stain).

Figure 1b: Higher magnification demonstrates that the majority of marrow
cellularity consists of immature mononuclear cells, which are most
pronounced in the bottom right half of this image. The residual cellularity
consists of predominantly erythroid progenitors and occasional hypolobated
megakaryocytes (hematoxylin and eosin stain).

Figure 1c: Immunohistochemistry performed on the core biopsy supports
the flow cytometric immunophenotype. Approximately 80% of the marrow
cellularity consists of blasts expressing CD34, Myeloperoxidase (MPO), and
terminal Deoxynucleotidyl Transferase (TdT). The blasts also co-expressed
Pax-5 (not pictured).
Flow cytometry on the bone marrow specimen revealed blast having abnormal co-expression of terminal deoxynucleotidyl transferase, myeloperoxidase, CD10 (variable), CD13 (very low on a minor subset), CD33 (low), CD 34 (strong uniform), CD 38 (decreased), CD45 (low to absent), cCD79a, CD123, and HLA-DR (decreased) with normal expression of CD19 and CD71 without CD2, CD3 (surface or cytoplasmic). The expression of multiple B-lineage markers with expression of the MPO was consistent with a Mixed Phenotype Acute Leukemia (MPAL) B/myeloid previously classified as a biphenotypic leukemia.
Molecular cytogenetics findings
In 2004, the initial cytogenetic analysis of the bone marrow which was performed by an outside institution showed a 46, XY, del (5) (q13q33) in 19 of the 20 metaphases analyzed. No FISH had been performed at that time.
At the time of diagnosis of the BAL in 2013, the cytogenetics analysis of the bone marrow showed two unrelated clones: neartetraploidy (Figure 2B) and diploidy with one abnormality showing an inverted chromosome 5 that also had an interstitial deletion (Figure 2A): 80-86, XXYY, -2, -3, -4, -7, -7, -9, +10, -16,-20,-20, +21 [cp7]/ 46, XY, der(5) inv(5) (p13q31) del (5)(q13q31) [4]/46, XY [3].

Figure 2A: Karyotype of a near-tetraploidy showing multiple losses and one
gain of chromosome 10.
The losses can vary between cells except for monosomy 7 which was
consistent in every near-tetraploidy.
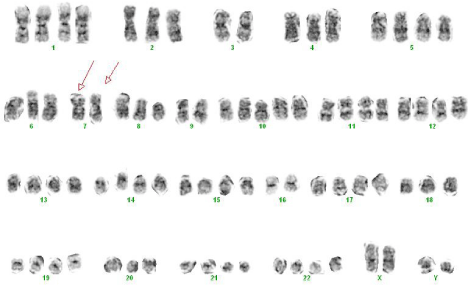
Figure 2B: A karyotype of a diploid clone showing 46,XY with a derivative
chromosome 5 showing an interstitial deletion and an inversion: der(5)inv(5)
(p13q31)del(5)(q13q31).
Initially, Fluorescence In-Situ Hybridization (FISH) of myelodysplastic syndrome probe panel was performed on 25 to 50 interphase cells hybridized with specific labeled fluorescent probes directed onto centromere chromosomes 8 3q26.2, 5q31, 7q31, 13q14 and 20q12. FISH results showed the following results: nuc ish (EVI1x3) [30/50], (D5S23, D5S721, EGR1)x4 [24/25], (D7Z1, D7S486)x2 [25/25], (CEP8x4, D20S108x2) [14/25], (FOXO1x3) [23/25]. These results indicate that EGR1 was not deleted despite the 5q deletion identified by conventional chromosome analysis. Therefore, FISH of D5S23/D5S721 and EGR1 were repeated on the abnormal diploid clone. FISH revealed that EGR1 was in the short arm of 5p and were on the long arm indicating the presence of an inversion (Figure 2C).

Figure 2C: Fluorescence in-situ hybridization using D5S23 (Spectrum
Green) and EGR1 (Spectrum Red) probes on abnormal diploid metaphase
cell revealed normal location of the probes on the normal chromosome 5
(arrows) and inverted location on the derivative chromosome 5: EGR1 on 5p
and D5S23 on 5q of the inversion 5 (arrow heads).
In order to rule out any submicroscopic deletions or duplications, an array-comparative genome hybridization (a-CGH) was performed. The a-CGH showed loss of copies of chromosomes 3, 7 (twice), 9, 12, 15, 16, 17, 19, 20 and 22 and gain of chromosome 10. However, the log2 ratio for chromosome 7 was the highest (>-0.5) (Figure 2D).

Figure 2D: Genome profiling of the bone marrow DNA showing all
chromosomes (X-Axis) from 1 to 22 and X and Y and their log2 ratios (Y-Axis)
of the plot. The log2 ratio for chromosome 7 was the most showing a loss at
more than -0.5.
The a-CGH provided the exact breakpoints of the interstitial deletion of 5q at 5q13 and5q31.1. The EGR1 gene was not within the deleted segment but rather right at the telomeric breakpoint.
Molecular studies on the bone marrow were negative for mutations of NPM1(by exon sequencing), CEBPA(by combination of PCR amplification, fragment length analysis and direct sequencing of DNA fragments) and FLT 3- ITD and FLT 3 D835 variant (by PCR). These were sent off as part of our institutions protocol for evaluation of a newly diagnosed leukemia. Once the diagnosis of BAL was made, the patient received induction chemotherapy for acute myeloid leukemia with cytarabine, idarubicin and cladrabine.
The bone marrow biopsy done at day fourteen showed a markedly hypocellular bone marrow with no morphologic evidence of residual acute leukemia. Minimal Residual Disease (MRD) analysis was negative on the day 14 bone marrow. The patient’s post-induction course was complicated by typhilitis and candida krusei fungemia and enterococcal bacteremia.
The bone marrow biopsy performed at the time of hematologic recovery revealed a variably hypocellular marrow with trilineage dyspoiesis, myeloid immaturity and 4% blast forms. Based on immunophenotypic analysis these blast forms were not consistent with residual acute leukemia. The patient was deemed to have achieved a complete remission (CR1).
He underwent consolidative reduced intensity double cord blood transplantation in CR1 conditioned with fludarabine, cyclophosphamide and total body irradiation. His posttransplantation course was complicated by skin and gut graft vs host disease which resolved with systemic corticosteroids. At his last clinical assessment, he was 396 days post-transplantation with no evidence of recurrent leukemia and off all immunosuppression.
Discussion
We report a case of leukemic transformation into a BAL (B-cell/ myeloid) associated with two unrelated cytogenetic clones in a patient with a history of 5q- MDS. In this case, a single blast population coexpressed the B-lymphoid (CD 10, CD 19, HLA-DR) and myeloid (CD13, CD33) antigens. B-cell/myeloid BAL accounts for more than 50% of BAL cases [1,2]. Among patients with BAL, there is a high incidence of cytogenetic abnormalities with only 13-15% having a normal karyotype [2,3]. The most common abnormality in BAL is the complex karyotype which frequently involves chromosomes 5, 7 and 6q [4]. Hyperdiploidy occurs relatively infrequently with a reported frequency of 3-7% in BAL [2,4].The present case is unique as it describes a patient with BAL with two cytogenetically unrelated abnormal clones: one near- tetraploid clone displaying monosomies 3, 7 and 16 (twice) and one diploid clone showing an inverted 5q that had an interstitial deletion not involving EGR1 probe.
BAL is associated with a poor outcome in terms of achieving Complete Responses (CR) and survival [5,6]. Based on results of adult and mixed adult/pediatric series patients with BAL have CR rates of 30-80.6% with median DFS of 5-12 months and overall survival of 6.5-30.3 months [7]. This may be associated with the high frequency of unfavorable karyotypes. Secondly, the optimal therapy of these patients is still not defined. There is as yet no consensus whether induction therapy should follow AML and/or ALL induction protocols, and whether hematopoeitic stem cell transplantation may be effective [1]. Matutes et al. in a retrospective review of 100 patients with MPAL found an 85% response rate of patients treated with ALL induction as opposed to 41% in patients treated with standard AML induction. This translated into a survival advantage in favor of ALL induction with a median overall survival of 139 months for patients treated with ALL protocols vs 11 months for patients treated with AML protocols (P=0.002) [8]. This data is consistent with the findings of Rubnitz et al. who studied 33 pediatric patients with MPAL. However, despite these finding Rubnitz et al. suggested using an AML based regimen as first line therapy and if a CR is not achieved to switch to ALL targeted treatment [9]. In our case CR was achieved with standard AML induction with cytarabine, idarubicin and cladribine and following a consolidative allogeneic transplantation the patient remains disease free thirteen months post-transplantation. Factors that have been associated with improved outcomes in BAL include B-myeloid phenotype [10], complete remission at the time of transplantation, younger age and use of an intensified conditioning regimen [11]. Our patient had 2 out of these four favorable risk factors. Furthermore the presence of a high-hyperdiploidy (>50 chromosomes) has in one pediatric retrospective study of 25 patients with BAL demonstrated a good treatment response and long-term overall survival even in patients treated with chemotherapy alone [12]. The hyperdiploid clonal cell population may be one of the factors driving this patient’s favorable outcome.
One of the clones of cells harbored a unique 5 q deletion. The most commonly observed interstitial deletions in AML/MDS are del(5) (q13q31), del(5)(q13q33), and del(5(q22q33), forming a Commonly Deleted Region (CDR) at 5q31-q32 [13]. This region contains the EGR 1 gene which protects normal cells from tumorogeneic transformation by inducing apoptosis or growth arrest upon DNA damage. In the 10% of patients with MDS/AML who harbor the 5q deletion, EGR1 is consistently lost [14]. In our case the diploid clone of cells had one structural aberration involving chromosome 5 which appeared inverted and deleted. The inversion breakpoints were at 5p13 and 5q31. The deletion of the inverted chromosome 5 had the breakpoints at 5q13 and 5q31. Although this is the typical deleted region in AML/MDS what is unique in this case is the lack of involvement of the EGR 1 gene locus and the formation of the inversion. However, gene mutation of EGR1 cannot be ruled out at this point. Furthermore, while there has been a reported case of MPAL associated with an inv (5) (q13q31) as the only abnormality [15] the complexity of the rearrangement seen in this case has to our knowledge never been reported [16].
While near- tetraploidy occurs at a frequency of 1-2% in cases of adult and childhood ALL and has been associated with a favorable prognosis [17], it is rarely found in AML. Iyer et al. in a case series of 11 patients with AML and 2 patients with MDS with massive hyperdiploidy and tetraploidy found that most of the patients were older males with de novo disease [18]. This is similar to the data published by the EGIL in a retrospective study of 25 cases of neartetraploid AML [4]. Our patient was an elderly male; however he did have a history of MDS. Massive hyperdiploidy and near tetraploidy in AML is associated with low remission rates, short survival and poor prognosis [5,19-21]. This is in contrast to the data published by Park et al. in a retrospective study of 25 pediatric cases of BAL in which patients with high-hyperdiploidy were found to have a good treatment response and long term survival [12]. In our case the patient achieved remission by morphological, and flow cytometric data using standard AML induction with cytarabine, idarubicin and cladrabine.
Another unique cytogenetic feature of this case is the presence of a monosomy 7 which is hidden by the near -tetraploidy (Figure 2A). This is evident in this case by the presence of 2 copies of chromosome 7 when 4 copies were expected given the presence of near- tetraploidy in the clone (Figure 2A). There were other chromosome losses except the a-CGH log2 ratio for chromosome 7 loss was the highest showing more than -0.5. This may indicate that monosomy 7 was found in every near-tetraploid clone indicative of a primary loss while the other chromosome losses could have been secondary. This may suggest that monosomy 7 occurred first and then followed by endoreduplication. To our knowledge only 5 other cases have been reported of a monosomy 7 in the setting of massive AML near- tetraploidy All of these five cases of AML showed minimal or no differentiation or were unclassifiable. This is consistent with the finding of Béné et al. in the retrospective study of 25 patients with near-tetraploid AML found that in 40% of cases the blast demonstrated undifferentiated morphology [4]. Our case is unique since it is only the first reported case of a near tetraploidy with a hidden monosomy 7 associated with a MPAL [16].
Conclusion
We described a case of two abnormal unrelated clones in a patient with BAL with CD markers coexistent on the same blast population in a patient with a history of MDS. In this patient we also described a unique 5q inversion/ deletion not involving EGR1. Despite the complexity of this patient’s tumor cytogenetics he had an excellent response to AML induction chemotherapy and a long relapse free survival. Since this is the first time that this 5 q inversion/deletion has been described in AML it is unknown whether it carries the same poor prognostic implication associated with 5q deletions with loss of EGR1 gene. This remains to be investigated with more cases of similar findings.
References
- Yan L, Ping N, Zhu M, Sun A, Xue Y, Ruan C, et al. Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica. 2012; 97: 1708-1712.
- Brunning RD, Matutes E, Borowitz M, et al. Acute leukemias of ambiguous lineage. ES, Harris NL, Stein H, Vardiman JW, editors. In: Jaffe World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haemopoietic and Lymphoid Tissues. Lyon, France: IARC Press. 2001; 106-107.
- Carbonell F, Swansbury J, Min T, Matutes E, Farahat N, Buccheri V, et al. Cytogenetic findings in acute biphenotypic leukemia. Leukemia. 1996; 10: 1283-1287.
- Bène MC, Castoldi G, Derolf A, Garand R, Haas T, Haferlach T, et al. Near-tetraploid acute myeloid leukemias: an EGIL retrospective study of 25 cases. Leukemia. 2006; 20: 725-728.
- Killiack S, Matutes E, Powles RL, Hamblin M, Swansbury J, Treleaven JG, et al. Outcome of biphenotypic acute leukemia. Haematologica. 1999; 84: 699-706.
- Legrand O, Perrot JY, Simonin G, Baudard M, Cadiou M, Blanc C, et al. Adult biphenotypic acute leukemia: an entity with poor prognosis which is related to unfavorable cytogenetics and p-glycoprotein over-expression. Br J Haemtol. 1998; 100: 147-155.
- Wolach O, Stone RM. How I treat mixed phenotype acute leukemia. Blood. 2015.
- Matutes E, Pickl WF, Veer M, Morilla R, Swansbury J, Strobl H, et al. Mixed-phenotype acute leukemia: clinical and laboratory features and outcome in 100 patients defined according to the WHO 2008. Blood. 2011; 117: 3163-3171.
- Rubnitz JE, Onciu M, Pounds S, Shurtleff S, Cao X, Raimondi SC, et al. Acute mixed lineage acute leukemia in children: the experience of St Jude Children’s Research Hospital. Blood. 2009; 113: 5083-5089.
- Lee JH, Min YH, Chung CW, Kim BK, Yoon HJ, Jo DY, et al. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008; 49: 700-709.
- Liu QF, Fan ZP, Wu MQ, Sun J, Wu XL, Xu D, et al. Allo-HSCT for acute leukemia of ambiguous lineage in adults: the comparison between standard conditioning and intensified condition regimens. Annals of Hematology. 2013; 92: 679-687.
- Park JA, Ghim TT, Bae KW, Im HJ, Jang SS, Park CJ, et al. Stem cell transplant in the treatment of childhood biphenotypic leukemia. Pediatr Blood Cancer. 2009; 53: 444-452.
- Van den Berghe H, Cassiman JJ, David G, Fryns JP, Michaux, JL, Sokal G. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature. 1974; 251:437-438.
- Bandyopadhyay R, Baron V. EGR 1 (early growth response 1). Atlas Genet Cytogenet Oncol Haematol. May 2010.
- Butcher BW, Wilson KS, Kroft SH, Collins RH, Bhushan V. Acute leukemia with B-lymphoid and myeloid differentiation associated with an inv (5) (q13q33) in an adult patient. Cancer Genet Cytogenet. 2005; 157: 62-6.
- Mitelman F, Johansson B, Mertens F, editors. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. 2013.
- Walters R, Kantarjian HM, Keating MJ, Estey EH, Trujillo J, Cork A, et al. The importance of cytogenetic studies in adult acute lymphocytic leukemia. Am J Med. 1990; 89: 579-587.
- Iyer RV, Sait SN, Matsui S, Block AW, Barcos M, Slack JL, et al. Massive hyperdiploidy and tetraploidy in acute myelocytic leukemia and myelodysplastic syndrome. Cancer Genet Cytogenet. 2004; 148: 29-34.
- Mrózek K, Heinonen K, de la Chapelle A, Bloomfield CD. Clinical significance of cytogenetics in acute myeloid leukemia. Semin Oncol. 1997; 24: 17-31.
- Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G, et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukemia Working Parties. Blood 1998; 92: 2322-2333.
- Byrd JC, Mrózek K, Dodge R, Carroll AJ, Edwards C, Pettenati MJ, et al. Pre-treatment cytogenetics predict initial induction success and overall survival in adult. Blood patients with de novo myeloid leukemia: results from CALGB 8641. Blood. 2001; 98: 457a.
Citation: Ramlal R, Lionberger JM, Robbins KJ and Batanian JR. Co-Existence of Two Abnormal Clones: Diploidy with a Unique Inverted 5q Deletion Not Involving EGR1 and Near-Tetraploidy with Monosomy 7 in Biphenotypic Leukemia Arising from Myelodysplastic Syndrome. Austin J Genet Genomic Res. 2015;2(1): 1009. ISSN : 2471-030X