
Review Article
Austin J Genet Genomic Res. 2015;2(1): 1010.
Chelating Drug Therapy: An Update
Vijay Kumar1, Ashok Kumar2*, Sandeep Kumar Singh1, Manoj Kumar3, Surendra Kumar2, Dinesh Kumar4 and Ragni Singh5
1Department of Neurology, SGPGIMS, India
2Department of Medical Genetics, SGPGIMS, India
3Department of Microbiology, SGPGIMS, India
4Department of Chemistry, Dr. R.M.L. Avadh University, India
5Bheem Rao Ambedkar Bihar University, India
*Corresponding author: Ashok Kumar, Department of Medical Genetics, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India
Received: March 12, 2015; Accepted: April 24, 2015; Published: April 27, 2015
Abstract
Purpose: To study the clinical effects of metal toxicity and current recommendations for management, including chelation therapy, are reviewed.
Summary: Metals are essential to many biological processes, but excess of it becomes hazardous to life. These are necessary for cell growth, electron transport chain, several enzymatic activities and response of immune systems. They also serve as a cofactor for several enzymes. Chelation therapy is used for clinical management of the excess of metal. However, each metal requires a specific chelation agent. A chelate is a compound form between metal and a compound that contains two or more potential ligands. A promising Fe chelator is Desferrioxamine (Desferal). Penicillamine and Trientine are uses for copper chelation. Meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto- Propanesulphonate (DMPS) can be used as effective chelator of mercury. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead.
Conclusion: Metal toxicity remains a significant public health concern. Elimination of elevated metal ions can be achieved by proper chelation agents. An inappropriate protocol of chelation therapy has the severe side effect which must be taken into consideration before chelation therapy.
Keywords: Chelating agent; Copper; Iron; Mercury; Lead; Cadmium
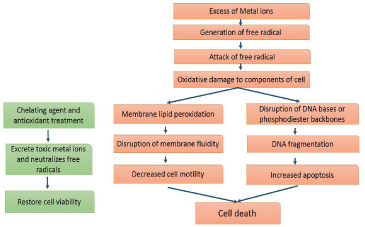
Figure 1: Graphical Abstract
Introduction
Metals are necessary for many biological processes. Many of these work as cofactor in many biological reactions. For example, copper, zinc and iron have an essential role in cell growth, oxygen utilization, various enzymatic activities and response of immune systems. Iron is found in hemoglobin and acts as a functional component for various electron transfer enzymes. Copper is needed for SOD, melanin and electron transport chain. Various proteins required zinc for folding, configurational changes, or activity. Zinc is also needed for DNA folding [1]. Metal ion transporters participate in maintaining the required levels of the various metal ions in the cellular compartments [2-4].
The failure of metal-ion homeostasis has been implicated in numerous diseases. Metal ions induce the generation of Reactive Oxygen Species (ROS) which leads to lipid peroxidation of the biological membrane. ROS may cause inhibition of oxidative phosphorylation, disruption of the electron transport system. These metals can interact with DNA and proteins causing oxidative deterioration of biological macromolecules. Increased amount of metal in the cytosol may disrupt the intracellular redox status, or may alter protein conformation and inhibit protein function; through metal substitution and interactions with sulfhydryl groups [5-12].
Metal Toxicity and Oxidative Stress
Homeostasis of metal ions, maintained through highly regulated mechanisms of uptake, storage and secretion [1]. Excess accumulation of any metals leads to adverse effect on cell function and integrity. Redox-active metals such as iron, copper, chromium and cobalt generate free radicals. Transition metal Copper and Iron is a redoxactive metal capable of catalyzing the formation of hydroxyl radicals via a Haber–Weiss or Fenton-like reaction. Excess of these metals often results in pathological conditions that have to be in conformity with intracellular oxidative damage. Metals are known to modulate gene expression by interfering with signal transduction pathways that play an important part in cell growth and development [13]. Actions of metals interfere with deregulation of cell proliferation by activating various transcription factors, controlling cell cycle progression and apoptosis and altered calcium and sulphydryl homeostasis [3,4,13]. Cadmium induces oxidative stress, by depleting intracellular antioxidants, such as glutathione, or inhibiting the activity of superoxide dismutase. Interruption of iron and copper homeostasis has proved to play a key role in the etiology of neurological disorders such as Alzheimer’s disease and Parkinson’s disease.
Reactive free radicals have the potential to interfere with cellular lipids, nucleic acids, carbohydrates and proteins which ultimately result in impairment in cellular function and integrity.
Redox-inert elements such as cadmium and arsenic have no known biological function and are even found to be toxic at low concentrations. The key path for their carcinogenicity and toxicity is the depletion of glutathione, bonding to sulphydryl groups of proteins.
Chelation Agent
To excrete the excess of metal, chelation therapy is to be used. Chelation is a chemical reaction in which metal is bounded to chelator by coordination bond. Organic ligand is called chelator or chelation agent. The chelate is a metal complex. Five and six membered chelate rings are the most stable, and polydentate chelators are more stable chelates than chelators with only one ligand atom. The stability of chelates varies with the metal and the ligand atoms. For example, lead and mercury have greater affinities for sulfur and nitrogen than for oxygen ligands; calcium, however, has a greater affinity for oxygen than for sulfur and nitrogen. These differences in affinity serve as a basis for selectivity of action of a chelation agent in the body.
An ideal chelating agent
Chelator should have the capability to form non-toxic complex with metal ions, have good water solubility, easily excreted from the body and able to enter the cell membrane. The chelation agent may be administered intravenously, intramuscularly, or orally, being dependent on the agent and the type of poisoning. It detoxify poisonous metal agents, such as mercury, arsenic, and lead, by converting them to a chemically inert form that can be excreted without further interaction with the body. It has higher affinity for toxic metals than for body. Should also have higher affinity for toxic metals than body ligands. Chelation drug was approved by the U.S. Food and Drug Administration in 1991 [14-25].
The effectiveness of a chelating agent
It depends on numerous factors: (a) the relative affinity of the chelator for the heavy metal as compared with essential body metals, (b) the distribution of the chelator in the body as compared with the distribution of the metal, (c) the capacity of the chelator to remove the metal from the body once chelated, (d)the capacity to retain chelation activity at the pH of body fluids, and (e) it must bind the metal more avidly than endogenous ligands [26-28].
Iron Toxicity and Chelation Therapy
Iron is an important mineral for normal cellular physiology, but an excess can cause cell injury. Iron may act as a catalyst for the initiation of free radical reactions. Fe is deposited in various internal organs, especially in the liver. The common symptoms and pathology are: hepatomegaly, subclinical inflammation, skin pigmentation, steatosis, insulin resistance, joint diseases and lethargy. A promising Fe chelator is Desferrioxamine (Desferal), Clioquinol and Aroylhydrazones. Desferrioxamine is incapable of crossing the blood-brain barrier (BBB), due to its size and hydrophobicity. Clioquinol, a small lipophilic chelator that can cross the BBB, has also been found to produce beneficial effects in patients with Alzheimer’s disease. However, clioquinol is not iron selective and has very toxic effects. Aroylhydrazones are the latest nontoxic lipophilic Fe chelators that can form a neutral complex with Fe and diffuse out of the membrane. Polyphenols compound may have antioxidant properties and can bind Fe. In beta-thalassemia patients who undergo regular transfusions, deferoxamine treatment has been found to be effective. Iron chelation therapy using iron (III) specific chelators such as desferrioxamine (DFO, Desferal), deferasirox (Exjade or ICL- 670), and deferiprone (Ferriprox or L1) are the current standard of care for the treatment of iron overload [17, 24,29-35,] (Table 1).
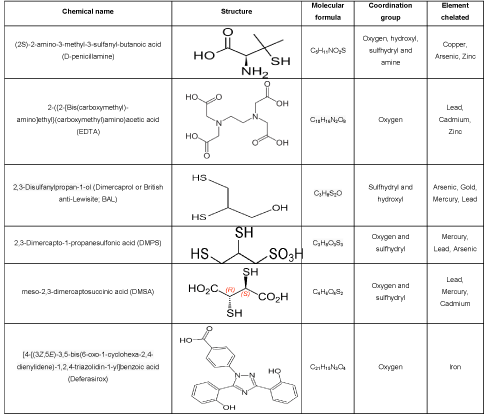
Table 1: List of common chelating agent used in metal toxicity.
Copper Toxicity and Chelation Therapy
Copper acts as a cofactor for many enzymes necessary for the redox reaction such as cytochrome c oxidase, ascorbic oxidase or superoxide dismutase. Copper is commonly used in the biological system for electron transport system [13,36]. Cu promotes oxidative damage in the conditions of increased Cu levels in the liver and brain. The best known disorder associated with Cu dyshomeostasis is Wilson’s disease, an autosomal recessive disorder linked to the Cu transporter expressed in hepatocytes. Cu toxicity has been linked with cardiovascular disease, atherosclerosis, diabetes, cancer progression and especially to neurological disorders. Copper can also induce oxidative stress by depleting glutathione levels [6,9,37,38]. D-Penicillamine has been extensively used in copper overload [39-42]. Trientine or tetrathiomolybdate has been increasingly recommended as the first-line treatment for neurologic Wilson disease [43-48] (Table 1).
Mercury Toxicity and Chelation Therapy
The clinical manifestation of mercury toxicity includes hypertension, myocardial infarction, reduction in heart rate, coronary heart disease and carotid obstruction, generalized atherosclerosis, renal dysfunction, proteinuria, an increase in total and cardiovascular mortality [49]. Chelators like meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto-Propanesulphonate (DMPS) can effectively excrete mercury into the urine. These drugs can be given orally and have relatively low toxicity compared to the classical antidote Dimercaptopropanol (BAL). BAL is a compound containing two –SH groups and is regarded as a preferred agent for arsenic, mercury, cadmium and other, metal toxicity. Dimercaprol competes with the thiol groups of enzymes for binding the arsenic or other metals to form a durable metal-chelate which is subsequently excreted by the body in the urine. On comparing the efficacy of the dithiol chelators in animals, DMSA was superior in removal of methylmercury, including animal brains. Although DMPS did not affect levels in the brain, it was patronizing at removing methylmercury from the kidney. In mice, cadmium was removed more effectively by DMSA than DMPS [49,50-53, 54] (Table 1).
Lead Toxicity and Chelation Therapy
Lead inhibits variety of body processes and is toxic to many organs and tissues including the heart, bones, intestines, kidneys and central nervous systems. It disrupts the development of the nervous system so it is particularly toxic to children, causing potentially permanent learning and behavior disorders. Symptoms include headache, anemia, abdominal pain, irritability, and in severe cases of seizures, coma, and death [23]. Dimercaprol, edetate calcium disodium, and succimer are the three agents primarily used for chelation of lead. Another effective chelator employed in the treatment of lead toxicity mentioned above is CaNa2EDTA. CaNa2EDTA chelates only extracellular lead it is most often used in conjunction with BAL to increase its efficiency [23,53,55-59] (Table 1).
Side Effect of Chelation Therapy
Chelation therapy can have adverse effects when used inappropriately. Various chelation agents may cause specific side effects if used improperly. When accurate protocol is to be carried out, there is a low occurrence of side effects. During the course of chelation therapy, it is important in order to be given the appropriate dose of drug. Because high dose of it is for longer duration may reduce the essential metal below the required threshold level. The most common side effects can include fever, headache, nausea, and vomiting. Serious side effects include heart failure; a sudden drop in blood pressure; permanent kidney damage; and bone marrow depression, diarrhea, convulsions or seizures, breathlessness or tightness in the chest, respiratory failure and low blood calcium [60]. Dimercaprol is toxic with a tendency to accumulate arsenic in some organs and exhibits side effects including nephrotoxicity and hypertension. The most adverse effect of CaNa2EDTA administration is the redistribution of lead to the brain. CaNa2EDTA causes renal toxicity and can deplete the body of essential minerals [61]. Dimercaptosuccinic Acid (DMSA) is an analogue of dimercaprol and is illustrated in the treatment of lead or arsenic poisoning in children [62,63]. A significant amount of patients treated with BAL experienced vomiting, fever, nausea and cardiological complications [62]. In the course of DMSA chelation therapy, in patients with chronic lead intoxication, hemolytic anemia has been reported [62].
Conclusion
In the present review, we give an update of the appropriate use of chelation agents in the treatment of intoxications by metals. Exposure to metals is a common phenomenon due to their environmental prevalence. Metal intoxication leads to the generation of reactive oxygen and nitrogen species. These metals have a high affinity for thiol groups containing enzymes and proteins, which are accountable for normal cellular defense mechanism. Long term exposure to these metals could be expected to result in apoptosis. Signaling components affected by metals include growth factor receptors, G-proteins, MAP kinases and transcription factors. Metal-mediated formation of free radicals also causes various modifications to DNA bases, enhanced lipid peroxidation, and altered calcium and sulfhydryl homeostasis. Lipid peroxides were formed by the attack of free radicals on polyunsaturated fatty acid residues of phospholipids.
Chelation therapy with chelation agents like Calcium Disodium Ethylenediamine Tetra Acetic Acid (CaNa(2)EDTA), British Anti Lewisite (BAL), sodium 2,3-dimercaptopropane 1-sulfonate (DMPS), meso 2,3-Dimercaptosuccinic Acid (DMSA) etc., is considered to be the best known treatment against metal poisoning. The clinical use of the old chelators EDTA (Ethylene Diamine Tetra Acetate) and BAL (2,3-dimercaptopropanol) is now limited due to the inconvenience of parenteral administration, their own toxicity and tendency to increase the neurotoxicity of several metals. The relatively new chelators meso-2,3-Dimercaptosuccinic Acid (DMSA) and 2,3-Dimercapto-Propanesulphonate (DMPS) can effectively mobilize deposits of mercury as well as of lead into the urine. These drugs can be managed orally and have relatively low toxicity compared to the classical antidote. DMSA and DMPS are less toxic and more efficient than BAL in the clinical treatment of heavy metal poisoning, and available as capsules for oral use. In copper overload, DMSA seems to be a potent antidote, although d-penicillamine is still widely used. In the chelation of iron, the thiols are inefficient, since iron has a higher affinity for ligands with nitrogen and oxygen, but the new oral iron antidotes deferiprone and desferasirox are useful.
The treatment with these chelation agents is compromised with a number of serious side-effects. Studies demonstrate that supplementation of antioxidants along-with a chelation agent proves to be a better treatment regimen than monotherapy with chelation agents. Combined chelation therapy treatment with antioxidant is useful in metal toxicity. One trial study showed a When antioxidants were combined with chelating agents it upgraded chelating ability. A combination of DMSA with alpha-lipoic acid in lead acetate exposed animals completely ameliorated the oxidative damage [64]. A similar effect of improved chelating ability was observed for CaNa2EDTA administrated in conjunction with zinc [65]. It seems that chelating agents used in combination with antioxidants can be a standard strategy in treatment of heavy metal toxicity. Structurally different chelators may be used in order to achieve a more effective removal of toxic metals [57]. Several new synthetic homologues and experimental chelating agents have been developed and tested in vivo for their metal binding effects.
References
- Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM. Metal ions in biological catalysis: from enzyme databases to general principles. Journal of biological inorganic chemistry. J Biol Inorg. 2008;13: 1205-1218.
- Rolfs A1, Hediger MA. Metal ion transporters in mammals: structure, function and pathological implications. J Physiol. 1999; 518: 1-12.
- Arlt S, Beisiegel U, Kontush A. Lipid peroxidation in neurodegeneration: new insights into Alzheimer's disease. Curr Opin Lipidol. 2002; 13: 289-294.
- Boveris A, Musacco-Sebio R, Ferrarotti N, Saporito-Magriñá C, Torti H, Massot F, et al. The acute toxicity of iron and copper: biomolecule oxidation and oxidative damage in rat liver. J Inorg Biochem. 2012; 116: 63-69.
- Halliwell B. Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging. 2001; 18: 685-716.
- Hayashi M, Fuse S, Endoh D, Horiguchi N, Nakayama K, Kon Y, et al. Accumulation of copper induces DNA strand breaks in brain cells of Long-Evans Cinnamon (LEC) rats, an animal model for human Wilson Disease. Exp Anim. 2006; 55: 419-426.
- Hayashi M, Kuge T, Endoh D, Nakayama K, Arikawa J, Takazawa A, et al. Hepatic copper accumulation induces DNA strand breaks in the liver cells of Long-Evans Cinnamon strain rats. Biochem Biophys Res Commun. 2000; 276: 174-178.
- Hayashi M, Kuge T, Endoh D, Nakayama K, Arikawa J, Takazawa A, et al. Hepatic iron accumulation is not directly associated with induction of DNA strand breaks in the liver cells of Long-Evans Cinnamon (LEC) rats. Exp Anim. 2002; 51: 43-48.
- Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J . Toxicity of copper on isolated liver mitochondria: impairment at complexes I, II, and IV leads to increased ROS production. Cell Biochem Biophys. 2014; 70: 367-381.
- Martin HL, Teismann P . Glutathione--a review on its role and significance in Parkinson's disease. FASEB J. 2009; 23: 3263-3272.
- Reddy PV, Rao KV, Norenberg MD. The mitochondrial permeability transition, and oxidative and nitrosative stress in the mechanism of copper toxicity in cultured neurons and astrocytes. Lab Invest. 2008; 88: 816-830.
- Reed TT. Lipid peroxidation and neurodegenerative disease. Free Radic Biol Med. 2011; 51: 1302-1319.
- Valko M1, Morris H, Cronin MT . Metals, toxicity and oxidative stress. See comment in PubMed Commons below Curr Med Chem. 2005; 12: 1161-1208.
- Bamonti F, Fulgenzi A, Novembrino C, Ferrero ME. Metal chelation therapy in rheumathoid arthritis: a case report. Successful management of rheumathoid arthritis by metal chelation therapy. Biometals. 2011; 24: 1093-8.
- Baxter AJ, Krenzelok EP. Pediatric fatality secondary to EDTA chelation. Clin Toxicol (Phila). 2008; 46: 1083-1084.
- Brent J. Commentary on the abuse of metal chelation therapy in patients with autism spectrum disorders. J Med Toxicol. 2013; 9: 370-372.
- Britton RS, Leicester KL, Bacon BR . Iron toxicity and chelation therapy. Int J Hematol. 2002; 76: 219-228.
- Budimir A. Metal ions, Alzheimer's disease and chelation therapy. Acta Pharm. 2011; 61: 1-14.
- Cuajungco MP, Fagét KY, Huang X, Tanzi RE, Bush AI. Metal chelation as a potential therapy for Alzheimer's disease. Ann N Y Acad Sci. 2000; 920: 292-304.
- Ernst E. Chelation therapy for peripheral arterial occlusive disease: a systematic review. Circulation. 1997; 96: 1031-1033.
- Flora SJ, Mittal M, Mehta A . Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res. 2008; 128: 501-523.
- Frizzell N, Baynes JW. Chelation therapy for the management of diabetic complications: a hypothesis and a proposal for clinical laboratory assessment of metal ion homeostasis in plasma. Clin Chem Lab Med. 2014; 52: 69-75.
- Gracia RC, Snodgrass WR . Lead toxicity and chelation therapy. Am J Health Syst Pharm. 2007; 64: 45-53.
- House E, Collingwood J, Khan A, Korchazkina O, Berthon G, Exley C. Aluminium, iron, zinc and copper influence the in vitro formation of amyloid fibrils of Abeta42 in a manner which may have consequences for metal chelation therapy in Alzheimer's disease. J Alzheimers Dis. 2004; 6: 291-301.
- Knudtson ML, Wyse DG, Galbraith PD, Brant R, Hildebrand K, Paterson D, et al. Chelation therapy for ischemic heart disease: a randomized controlled trial. JAMA. 2002; 287: 481-486.
- Nurchi VM, Alonso MC, Toso L, Lachowicz JI, Crisponi G. Chelation therapy for metal intoxication: comments from a thermodynamic viewpoint. Mini Rev Med Chem. 2013; 13: 1541-1549.
- Rahman YE. Potential of the liposomal approach to metal chelation therapy. Front Biol. 1979; 48: 625-652.
- Seely DM, Wu P, Mills EJ. EDTA chelation therapy for cardiovascular disease: a systematic review. BMC Cardiovasc Disord. 2005; 5: 32.
- Das P, Mukhopadhyay S, Mandal S, Chakraborty A, Pal A, Sarkar NK, et al. Acute toxicity test of a natural iron chelator and an antioxidant, extracted from Triticum aestivum Linn. (wheat grass). Nat Prod Res. 2014; 28: 1379-1382.
- Mladenka P, Kalinowski DS, Haskova P, Bobrovova Z, Hrdina R, Simunek T, et al. The novel iron chelator, 2-pyridylcarboxaldehyde 2-thiophenecarboxyl hydrazone, reduces catecholamine-mediated myocardial toxicity. Chem Res Toxicol. 2009; 22: 208-217.
- Roth JA, Feng L, Dolan KG, Lis A, Garrick MD. Effect of the iron chelator desferrioxamine on manganese-induced toxicity of rat pheochromocytoma (PC12) cells. J Neurosci Res. 2002; 68: 76-83.
- Santiago M, Matarredona ER, Granero L, Cano J, Machado A. Neuroprotective effect of the iron chelator desferrioxamine against MPP+ toxicity on striatal dopaminergic terminals. J Neurochem. 1997; 68: 732-738.
- Sarkar R, Hazra B, Mandal N. Amelioration of iron overload-induced liver toxicity by a potent antioxidant and iron chelator, Emblica officinalis Gaertn. Toxicol Ind Health. 2013.
- Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One. 2014; 9: e85115.
- Wong A, Alder V, Robertson D, Papadimitriou J, Maserei J, Berdoukas V, et al. Liver iron depletion and toxicity of the iron chelator deferiprone (L1, CP20) in the guinea pig. Biometals. 1997; 10: 247-256.
- Shim H, Harris ZL. Genetic defects in copper metabolism. J Nutr. 2003; 133: 1527S-31S.
- Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003; 189: 147-163.
- Kumar V, Kalita J, Misra UK, Bora HK. A study of dose response and organ susceptibility of copper toxicity in a rat model. J Trace Elem Med Biol. 2015; 29: 269-274.
- Brewer GJ, Terry CA, Aisen AM, Hill GM. Worsening of neurologic syndrome in patients with Wilson's disease with initial penicillamine therapy. Arch Neurol. 1987; 44: 490-493.
- Chen DB, Feng L, Lin XP, Zhang W, Li FR, Liang XL, et al. Penicillamine increases free copper and enhances oxidative stress in the brain of toxic milk mice. PLoS One. 2012; 7: e37709.
- Grazyna G, Agata K, Adam P, Tomasz L, Agata WC, Karolina D, et al. Treatment with D-penicillamine or zinc sulphate affects copper metabolism and improves but not normalizes antioxidant capacity parameters in Wilson disease. Biometals. 2014; 27: 207-215.
- Kalita J, Kumar V, Chandra S, Kumar B, Misra UK . Worsening of Wilson disease following penicillamine therapy. Eur Neurol. 2014; 71: 126-131.
- Cui Z, Lockman PR, Atwood CS, Hsu CH, Gupte A, Allen DD, et al. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer's and other CNS diseases. Eur J Pharm Biopharm. 2005; 59: 263-272.
- Gupte A, Mumper RJ. Copper chelation by D-penicillamine generates reactive oxygen species that are cytotoxic to human leukemia and breast cancer cells. Free Radic Biol Med. 2007; 43: 1271-1278.
- Walshe JM. Copper chelation in patients with Wilson's disease. A comparison of penicillamine and triethylene tetramine dihydrochloride. Q J Med. 1973; 42: 441-452.
- Brewer GJ, Askari F, Dick RB, Sitterly J, Fink JK, Carlson M, et al. Treatment of Wilson's disease with tetrathiomolybdate: V. Control of free copper by tetrathiomolybdate and a comparison with trientine. Transl Res. 2009; 154: 70-77.
- Brewer GJ, Askari F, Lorincz MT, Carlson M, Schilsky M, Kluin KJ, et al. Treatment of Wilson disease with ammonium tetrathiomolybdate: IV. Comparison of tetrathiomolybdate and trientine in a double-blind study of treatment of the neurologic presentation of Wilson disease. Arch Neurol. 2006; 63: 521-527.
- Hayashi M, Miyane K, Senou M, Endoh D, Higuchi H, Nagahata H, et al. Inhibitory effects of trientine, a copper-chelating agent, on induction of DNA strand breaks in kidney cells of Long-Evans Cinnamon (LEC) rats. Exp Anim. 2005; 54: 403-12.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012; 2012: 460508.
- Adams JB, Baral M, Geis E, Mitchell J, Ingram J, Hensley A, et al. Safety and efficacy of oral DMSA therapy for children with autism spectrum disorders: part B - behavioral results. BMC clinical pharmacology. 2009; 9: 17.
- Aposhian HV. DMSA and DMPS--water soluble antidotes for heavy metal poisoning. Annu Rev Pharmacol Toxicol. 1983; 23: 193-215.
- Blaucok-Busch E, Amin OR, Dessoki HH, Rabah T. Efficacy of DMSA Therapy in a Sample of Arab Children with Autistic Spectrum Disorder. Maedica (Buchar). 2012; 7: 214-221.
- Chisolm JJ. Safety and efficacy of meso-2,3-dimercaptosuccinic acid (DMSA) in children with elevated blood lead concentrations. J Toxicol Clin Toxicol. 2000; 38: 365-375.
- Kostyniak PJ, Soiefer AI. A methylmercury toxicity model to test for possible adverse effects resulting from chelating agent therapy. J Appl Toxicol. 1984; 4: 206-210.
- Flora G, Gupta D, Tiwari A. Toxicity of lead: A review with recent updates. Interdiscip Toxicol. 2012; 5: 47-58.
- Ambrus CM, Anthone S, Stadler A, Cameron MS, Wells K, Stadler I, et al. Treatment of lead poisoning with an immobilized chelator comparison with conventional therapy. Res Commun Mol Pathol Pharmacol. 2001; 110: 253-63.
- Kalia K, Flora SJ. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health. 2005; 47: 1-21.
- Schumacher HR Jr, Osterman AL, Choi SJ, Weisz PB. Calcinosis at the site of leakage from extravasation of calcium disodium edetate intravenous chelator therapy in a child with lead poisoning. Clin Orthop Relat Res. 1987; 219: 221-225.
- Victery W, Miller CR, Goyer RA . Essential trace metal excretion from rats with lead exposure and during chelation therapy. J Lab Clin Med. 1986; 107: 129-135.
- Blanusa M, Varnai VM, Piasek M, Kostial K . Chelators as antidotes of metal toxicity: therapeutic and experimental aspects. Curr Med Chem. 2005; 12: 2771-2794.
- Aposhian HV, Maiorino RM, Gonzalez-Ramirez D, Zuniga-Charles M, Xu Z, Hurlbut KM, et al. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology. 1995; 97: 23-38.
- Andersen O, Aaseth J. Molecular mechanisms of in vivo metal chelation: implications for clinical treatment of metal intoxications. Environl Health Perspect. 2002;110: 887-90.
- Bradberry S, Vale A. Dimercaptosuccinic acid (succimer; DMSA) in inorganic lead poisoning. Clin Toxicol (Phila). 2009; 47: 617-631.
- Sivaprasad R, Nagaraj M, Varalakshmi P. Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J Nutr Biochem. 2004; 15: 18-23.
- Batra N, Nehru B, Bansal MP. The effect of zinc supplementation on the effects of lead on the rat testis. Reprod Toxicol. 1998; 12: 535-540.