
Special Article: Dementia
Gerontol Geriatr Res. 2024; 10(1): 1098.
The Role of Nutrients on The Treatment of Sarcopenia and Muscles Across Age
Samer Yones*
Department of Pharmacy, Tartous University, Syria
*Corresponding author: Samer Yones Department of Pharmacy, Tartous University, Syria. Email: yonessamer22@gmail.com
Received: April 12, 2024 Accepted: May 07, 2024 Published: May 14, 2024
Abstract
Sarcopenia is a degenerative skeletal muscle condition associated with aging, resulting in muscle mass and function decline. It has been connected to inflammation, oxidative stress, insulin resistance, hormonal changes, and impaired muscle satellite cell activation. The gut microbiome is crucial for muscle health, and supplements like probiotics, prebiotics, protein, creatine, and beta-alanine can aid muscle growth and function while supporting gut health. Chronic low-grade inflammation is a primary cause of sarcopenia, activating pathways leading to muscle wasting and reduced protein synthesis. Insulin resistance, hormonal changes, impaired muscle satellite cell activation, and high fat mass levels also contribute to sarcopenia development. Resistance exercise and dietary supplements have proven effective in treating sarcopenia. Furthermore, a combination of resistance exercise and supplementation has shown to have a more significant positive impact on anthropometric and muscle function parameters, reducing the sarcopenic state. Therefore, understanding the gut microbiome’s role in muscle metabolism is essential for developing new sarcopenia treatments for all age groups.
Keywords: Nutrients supplements; Sarcopenia; Muscle mass
Introduction
Sarcopenia is a multifaceted age-related condition influenced by various mechanisms that result in decreased muscle strength and function due to impaired muscle synthesis and increased muscle catabolism [1]. These mechanisms include inflammation, oxidative stress, insulin resistance, hormonal changes, and inhibited activation of muscle satellite cells, among others, which are considered pivotal factors in the development and progression of sarcopenia [2,3]. Nutritional status plays a significant role in maintaining muscle structure and metabolism in individuals with sarcopenia, as chronic inflammation has been associated with both muscle dysfunction and gut metabolism [4]. Certain supplements, such as omega-3 fatty acids, have been shown to reduce systemic inflammatory markers and support gut and muscle health. The structure of the gut and changes in microbiota are closely linked to human health and disease as individuals age. Nutritional supplements containing essential nutrients can play a crucial role in promoting gut and muscle health [5-8]. For instance, probiotics and prebiotics can help maintain a healthy gut microbiome by fostering the growth of beneficial gut bacteria and enhancing gut microbial diversity [9]. Moreover, specific nutrients like protein, creatine, and beta-alanine have demonstrated benefits in supporting muscle growth and function. Furthermore, a recent study indicated that supplementing to restore gut balance in critically ill patients can lead to a shorter stay in the Intensive Care Unit (ICU), reduced muscle protein breakdown, and decreased complications from infections [10]. m,kij78y6Furthermore, a recent systematic analysis indicated that the gut microbiota could have a notable impact on muscle balance through the gut-muscle connection. Consequently, comprehending the correlation between gut microbiota and muscle function is crucial for the advancement of novel therapies for conditions like sarcopenia, particularly in different age groups [11].
Methods
We conducted a review by searching the Google Scholar, PubMed, and Directory Open access Journal databases for relevant information using keywords such as Nutrients, muscle mass, Nutrition, nutrient supplements, sarcopenia, microbiota, muscle strength, Insulin, Insulin resistance, vitamins, micronutrients, vitamins supplements, to identify primary comparative studies on treatment and management options for sarcopenia. The quality and strength levels of the results were considered and when available meta-analyses and systematic reviews, large epidemiological studies and randomized control trials represented the main source of data.
Results
Sarcopenia
Sarcopenia, a prevalent condition among the elderly, is a progressive disorder characterized by the loss of muscle mass and function. This condition is associated with various negative outcomes, including an increased risk of falls, functional decline, frailty, and mortality in older populations [12]. The European Working Group on Sarcopenia in Older People (EWGSOP) has established specific criteria for diagnosing sarcopenia, which involves assessing muscle mass, muscle strength, and physical performance using various measurement techniques [13-15]. Chronic low-grade inflammation has been identified as a potential mechanism contributing to muscle wasting in sarcopenia, as it can activate signaling pathways that lead to muscle degradation and reduce muscle protein synthesis [15]. Additionally, oxidative stress and insulin resistance have also been implicated in the development of sarcopenia, as they can disrupt the balance between muscle protein synthesis and degradation. Hormonal changes, such as decreased testosterone levels in men and decreased estrogen levels in women, may further contribute to sarcopenia by reducing muscle protein synthesis and increasing muscle protein degradation. Furthermore, the impaired activation of muscle satellite cells with aging can lead to decreased muscle repair and increased muscle wasting [3,12]. Body composition, specifically the presence of excess fat mass, has also been linked to sarcopenia, as studies have shown that increased fat mass is associated with decreased muscle mass and strength in older adults. This inverse relationship between fat mass and muscle mass may partially explain the pathogenesis of sarcopenia [16,17] (Figure 1 & 2).
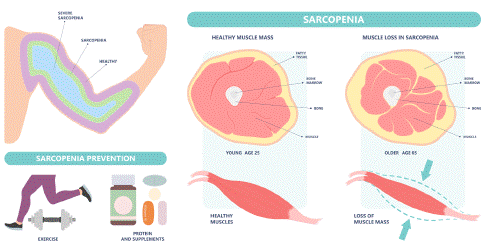
Figure 1: Sarcopenia.
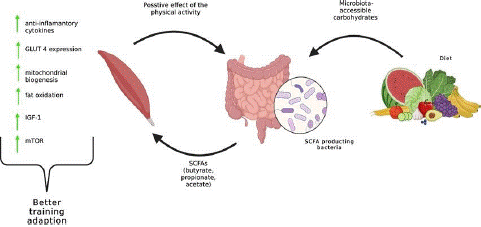
Figure 2: The role of Nutrition on the treatment of Sarcopenia.
Moreover, elevated levels of fat mass have been demonstrated to hinder insulin sensitivity, resulting in chronic low-grade inflammation, which can further contribute to the onset of sarcopenia [16,17]. Furthermore, fat mass has been proven to negatively impact muscle function by disrupting the mechanical signaling pathways necessary for muscle growth and maintenance. This disruption can lead to reduced muscle mass and strength, ultimately leading to the development of sarcopenia [16,17]. In terms of cellular changes, the age-related decline in skeletal muscle mass that may lead to sarcopenia is attributed to a reduction in myofiber size and number, affecting both fast and slow type myofibers, although the loss of fast myofibers (glycolytic metabolism) typically begins earlier [18]. Additionally, the function of the nervous system, which plays a crucial role in muscle strength, deteriorates with age due to the loss of motoneurons, axonal demyelination, and the retraction of nerve terminals from neuromuscular junctions [1,2]. An additional significant factor contributing to sarcopenia is the anabolic resistance of older skeletal muscle to protein nutrition, which can be improved through resistance exercise and dietary supplementation [19,20] The exploration of therapeutic strategies to mitigate the effects of sarcopenia is imperative. In this regard, non-pharmacological interventions such as nutritional supplementation with or without resistance exercise have been shown to mitigate age-related alterations in muscle structure. A recent systematic review indicated that strength-resistance training, either alone or in combination with aerobic exercise, has significantly positive effects on anthropometric and muscle function parameters, resulting in a reduction in sarcopenic conditions [21,22].
Nevertheless, the correlation between a regular physical exercise routine and supplementation is considered a more effective approach due to the significant role that nutrition plays in muscle maintenance. A randomized controlled trial was conducted to examine the impact of supplementation, specifically 32.4 g of whey protein, compared to a control group over a period of 12 weeks. The trial involved 115 male and female subjects over the age of 60 who also followed a 30-minute home-based resistance exercise program. The results indicated that the group receiving whey protein supplementation experienced a notable increase in grip strength, gait speed, and the time taken to complete chair stands [23].
Furthermore, a study explored the effects of a supplement containing minerals, Silybum marianum, and yeast ß-glucan derived from non-dairy bacteria. This supplement, which exhibited prebiotic properties, was administered to sedentary mice following a nonfat diet. The findings revealed an increase in lean mass among the mice [24].
In the context of sarcopenia, another study investigated 112 subjects with sarcopenia who received nutritional supplementation for 12 weeks. The supplementation consisted of 10g of whey protein and 800 IU of Vitamin D3, either with or without a resistance exercise program. The study compared these groups to an exercise-only group and a control group. The authors concluded that the combination of exercise and whey protein supplementation significantly improved appendicular muscle mass in sarcopenic adults [7].
Studies focusing on the impact of nutrition have demonstrated that a higher intake of minerals, such as calcium, is associated with the regulation of signaling pathways for muscle fibers [25]. A cross-sectional analysis involving 396,283 participants from the United Kingdom revealed that a higher intake of both calcium and magnesium was linked to a reduced likelihood of sarcopenia [26]. Additionally, a study conducted on 1339 older Korean adults found a positive correlation between daily calcium intake and appendicular skeletal muscle mass [27].
The data indicates that nutritional intervention could have a significant impact on age-related muscle changes and pathological changes associated with sarcopenia. Nevertheless, comprehending the physiological alterations in gut microbiota and its metabolism is crucial for effectively supplementing the diets of elderly individuals.
The Relationship between Gut Microbiota and Muscle
Although the surface area of gut villi decreases with age, bacterial cells within the gut do not undergo aging. However, as individuals age, they may encounter comorbidities linked to the metabolism of gut microbiota [28-30]. This occurrence could be associated with dietary habits since aging often coincides with a decrease in the consumption of fiber-rich foods and an elevated risk of malnutrition. Moreover, a reduced intake of fiber can result in a decline in the diversity of core microbiota, which could have adverse effects on gut health. Core microbiota refers to the taxa that are present in the majority of individuals in significant proportions, such as Bacteroidetes and Firmicutes in adults. Nevertheless, an improper diet might disrupt the balance of gut microbiota, leading to compromised nutrient absorption and the production of harmful bacterial byproducts, potentially contributing to the development of various diseases like sarcopenia [31-34]. The Gut-Muscle Axis theory suggests that micronutrients and metabolites derived from gut microbiota can influence muscle metabolism [11,35]. Recent studies have indicated that modifying this axis through interventions like supplementation could potentially reverse the effects of sarcopenia [36]. For instance, research on aged mice demonstrated that supplements containing Lactobacillus and Bifidobacterium notably improved muscle mass, strength, and endurance [37]. Furthermore, a clinical trial revealed that older individuals could benefit from the Gut-Muscle Axis pathways by consuming a prebiotic formulation comprising inulin and fructooligosaccharides. Therefore, exploring the impact of nutritional supplementation on the Gut-Muscle Axis could present a promising approach to delaying age-related muscle wasting and dysfunction [38,39].
The influence of Nutritional Supplements on Gut-Muscle Axis
The gut microbiome has a significant impact on the gut-muscle axis. In cases where there is a lack of microbiota homeostasis, harmful bacterial metabolites such as indoxyl sulfate and lipopolysaccharide can lead to bacterial depletion [11]. This depletion triggers a series of molecular pathways involving phosphoinositide-3-kinase/protein kinase B (PI3K/AKT), nuclear factor kappa B (NF-κB), and mitogen-activated protein kinases, ultimately resulting in muscle atrophy [40-46]. Furthermore, the upregulation of genes encoding E3 ubiquitin ligases Atrogin-1/MAFbx and Muscle RING Finger-1 (MuRF-1), as well as inflammatory cytokines, is observed. Activation of adenosine-5'-monophosphate–Activated Protein Kinase (AMPK), Forkhead box O3 (FoxO3), Atrogin-1/MuRF1 cascade (AMPK–FoxO3–Atrogin-1/MuRF1), and Branched-Chain Amino Acids (BCAA) catabolism is also noted in cases of bacteria depletion [11]. These activations can lead to reduced expressions of Insulin-like Growth Factor 1 (IGF1), myogenin, and myoblast determination protein 1, along with an increase in myostatin expression. Collectively, these pathways have a detrimental impact on the neuromuscular junction and mitochondrial metabolism, ultimately resulting in decreased muscle mass [40-46].
In this particular situation, the addition of probiotic bacteria has the potential to enhance both gut and muscle health. Consequently, several probiotic strains are commonly utilized in supplementary therapy [47,48]. These include:
- Lactobacillus acidophilus, which is known for its ability to improve gut health and boost the immune system.
- Another strain, Bifidobacterium bifidum, naturally resides in the gut and aids in improving digestive function and regulating the immune system.
- Lactobacillus rhamnosus, on the other hand, has been proven to reduce inflammation, promote gut health, and facilitate muscle recovery following exercise.
- Additionally, Streptococcus thermophilus has demonstrated its effectiveness in improving gut health and modulating the immune system.
When it comes to nutritional supplementation, scientific literature indicates a strong correlation between certain nutrients and minerals and the maintenance of the gut-muscle axis [6,47,48]. Consequently, the most crucial nutrients and minerals for this axis are protein and vitamin D.
- Adequate protein intake is vital for muscle growth and recovery, while
- Vitamin D plays a significant role in muscle function and also aids in modulating the immune system.
- Magnesium is a vital nutrient for muscle function and has the potential to reduce systemic inflammation.
- Omega-3 fatty acids, on the other hand, are essential fatty acids that play a role in maintaining the health of both the gut and muscles. They have been proven to enhance gut microbiota diversity, support gut barrier function, improve muscle function, and reduce muscle wasting.
- Prebiotics, which are indigestible fiber compounds, act as nourishment for beneficial gut bacteria. By consuming prebiotics, one can promote the growth of these beneficial bacteria and improve gut health, ultimately supporting muscle health. These components have demonstrated the ability to suppress glucocorticoid receptor and excessive AMPK activation, decrease inflammatory levels, repair mitochondria and neuromuscular junctions, and increase the expression of muscle growth-related genes such as IGF1, myogenin, and salt inducible kinase 1 [39,45,49,50].
They also synergistically modulate the PPAR coactivator 1a (Pgc-1a), which is involved in mitochondrial biogenesis, when combined with yeast ß-glucan, prebiotics, minerals, and Silybum marianum. In conclusion, a well-balanced and diverse diet that includes sufficient amounts of nutrients, minerals, and probiotics can enhance both gut and muscle health and regulate the gut-muscle axis. However, further research and clinical studies with different nutritional compositions and dosages are necessary to effectively modulate the Gut-Muscle Axis and prevent severe sarcopenic cases in the elderly population [51].
Conclusion
This review highlights the significance of nutritional supplementation in maintaining muscle homeostasis through the Gut-Muscle Axis. It emphasizes the need to investigate the impact of nutritional supplements on this axis across different age groups for various reasons. Firstly, as individuals age, there is a decline in both gut and muscle health, which can greatly affect overall well-being. Therefore, it is crucial to understand how nutritional supplements can modulate the gut-muscle axis in older adults to develop effective interventions that enhance gut and muscle integrity in this population. Secondly, the gut microbiome undergoes complex changes throughout life, and these changes can significantly impact gut and muscle health. Therefore, it is important to study the effects of nutritional supplements on the gut microbiome at different ages to develop targeted interventions that improve gut and muscle health. Thirdly, the efficacy of nutritional supplements can vary depending on age, as well as other factors such as health status and lifestyle. For instance, older adults may require higher doses of certain nutrients compared to younger adults, and the effects of certain nutrients may differ between these age groups. Investigating the role of nutritional supplements on the gut-muscle axis across age is essential for understanding these differences and developing interventions that effectively enhance gut and muscle health in older adults. Lastly, the gut-muscle axis is a complex system influenced by various factors including diet, exercise, and the composition of the gut microbiome. Therefore, comprehending the role of nutritional supplements in modulating this axis across different age groups is crucial for developing comprehensive and effective interventions that improve gut and muscle health. Hence, additional research is required to clarify the impact of various supplements, prebiotics, and probiotics, as well as the dosage effect, on the severity of sarcopenia. This necessitates conducting experimental studies utilizing molecular techniques and clinical trials employing standardized cut-off values (e.g. EWGSOP) to assess muscle structure and function.
References
- Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PloS One. 2011; 6: e28090.
- Ham DJ, Börsch A, Lin S, Thürkauf M, Weihrauch M, Reinhard JR, et al. The neuromuscular junction is a focal point of mTORC1 signaling in sarcopenia. Nat Commun. 2020; 11: 1-21.
- Marcell, TJ. Sarcopenia: Causes, consequences, and preventions. J Gerontol A Biol Sci Med Sci. 2003; 58: M911-M916.
- Chaiyasut C, Sivamaruthi BS, Lailerd N, Sirilun S, Khongtan S, Fukngoen P, et al. Probiotics supplementation improves intestinal permeability, obesity index and metabolic biomarkers in elderly Thai subjects: a randomized controlled trial. Foods. 2022; 11: 268.
- Calero CQ, Rincón EO, Marqueta PM. Probiotics, prebiotics and synbiotics: useful for athletes and active individuals? A systematic review. Benef Microbes. 2020; 11: 135-149.
- Castro EM, Murphy CH, Roche HM. Targeting the gut microbiota to improve dietary protein efficacy to mitigate sarcopenia. Front Nutr. 2021; 8: 656730.
- Yamada M, Kimura Y, Ishiyama D, Nishio N, Otobe Y, Tanaka T, et al. Synergistic effect of bodyweight resistance exercise and protein supplementation on skeletal muscle in sarcopenic or dynapenic older adults. Geriatr Gerontol Int. 2019; 19: 429-437.
- Kim S, Jazwinski SM. The gut microbiota and healthy aging: a mini-review. Gerontology. 2018; 64: 513-520.
- Mangiola F, Nicoletti A, Gasbarrini A, Ponziani FR. Gut microbiota and aging. Eur Rev Med Pharmacol Sci. 2018; 22: 7404-7413.
- Seifi N, Safarian M, Nematy M, Rezvani R, Khadem-Rezaian M, Sedaghat A. Effects of synbiotic supplementation on energy and macronutrients homeostasis and muscle wasting of critical care patients: study protocol and a review of previous studies. Trials. 2020; 21: 221.
- Liu C, Cheung WH, Li J, Chow SKH, Yu J, Wong SH, et al. Understanding the gut microbiota and sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. 2021; 12: 1393-1407.
- Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019; 393: 2636-2646.
- Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012; 31: 652-658.
- Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E: Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014; 6: 192.
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019; 48: 16-31.
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, et al. Relationship of interleukin-6 and tumor necrosis factor-a with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002; 57: M326-M332.
- Li CW, Yu K, Shyh-Chang N, Jiang Z, Liu T, Ma S, et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022; 13: 781-794.
- Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983; 6: 588-595.
- Farnfield MM, Breen L, Carey KA, Garnham A, Cameron-Smith D. Activation of mTOR signalling in young and old human skeletal muscle in response to combined resistance exercise and whey protein ingestion. Appl Physiol Nutr Metab. 2012; 37: 21-30.
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010; 95: 139-159.
- Denison HJ, Cooper C, Sayer AA, Robinson SM. Prevention and optimal management of sarcopenia: a review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin Interv Aging. 2015; 10: 859-869.
- Barajas-Galindo DE, Arnáiz EG, Vicente PF, Ballesteros-Pomar MD. Effects of physical exercise in sarcopenia. A systematic review. Endocrinol Diabetes Nutr (Engl Ed). 2021; 68: 159-169.
- Kang L, Gao Y, Liu X, Liang Y, Chen Y, Liang Y, et al. Effects of whey protein nutritional supplement on muscle function among community-dwelling frail older people: A multicenter study in China. Arch Gerontol Geriatr. 2019; 83: 7-12.
- Nehmi VA, Murata GM, de Moraes RCM, Lima GCA, De Miranda DA, Radloff K, et al. A novel supplement with yeast ß-glucan, prebiotic, minerals and Silybum marianum synergistically modulates metabolic and inflammatory pathways and improves steatosis in obese mice. J Integr Med. 2021; 19: 439-450.
- Berchtold MW, Brinkmeier H, Muntener M. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev. 2000; 80: 1215-1265.
- Petermann-Rocha F, Chen M, Gray SR, Ho FK, Pell JP, Celis-Morales C. Factors associated with sarcopenia: a cross-sectional analysis using UK Biobank. Maturitas. 2020; 133: 60-67.
- Seo MH, Kim MK, Park SE, Rhee EJ, Park CY, Lee WY, et al. The association between daily calcium intake and sarcopenia in older, non-obese Korean adults: the fourth Korea National Health and Nutrition Examination Survey (KNHANES IV) 2009. Endocr J. 2013; 60: 679-686.
- Thomson ABR, Keelan M. The aging gut. Can J Physiol Pharmacol. 1986; 64: 30-38.
- O’Toole PW, Jeffery IB. Gut microbiota and aging. Science. 2015; 350: 1214-1215.
- Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019; 16: 35-56.
- Claesson MJ, Jeffery IB, Conde S, Power SE, O’connor EM, Cusack S, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012; 488: 178-184.
- Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012; 9: 577-589.
- Bakhtiar SM, LeBlanc JG, Salvucci E, Ali A, Martin R, Langella P, et al. Implications of the human microbiome in inflammatory bowel diseases. FEMS Microbiol Lett. 2013; 342: 10-17.
- Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Reddy DN. Role of the normal gut microbiota. World J Gastroenterol. 2015; 21: 8787-8803.
- Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013; 28: 9-17.
- Bindels LB, Delzenne NM. Muscle wasting: the gut microbiota as a new therapeutic target?. Int J Biochem Cell Biol. 2013; 45: 2186-2190.
- Picca A, Fanelli F, Calvani R, Mulè G, Pesce V, Sisto A, et al. Gut dysbiosis and muscle aging: searching for novel targets against sarcopenia. Mediators Inflamm. 2018; 7026198.
- Buigues C, Fernández-Garrido J, Pruimboom L, Hoogland AJ, Navarro-Martínez R, Martínez-Martínez M, et al. Effect of a prebiotic formulation on frailty syndrome: a randomized, double-blind clinical trial. Int J Mol Sci. 2016; 17: 932.
- Ni Y, Yang X, Zheng L, Wang Z, Wu L, Jiang J, et al. Lactobacillus and Bifidobacterium improves physiological function and cognitive ability in aged mice by the regulation of gut microbiota. Mol Nutr Food Res. 2019; 63: 1900603.
- Doyle A, Zhang G, Fattah EAA, Eissa NT, Li YP. Toll-like receptor 4 mediates lipopolysaccharide-induced muscle catabolism via coordinate activation of ubiquitin-proteasome and autophagy- lysosome pathways. FASEB J. 2011; 25: 99-110.
- Enoki Y, Watanabe H, Arake R, Sugimoto R, Imafuku T, Tominaga Y, et al. Indoxyl sulfate potentiates skeletal muscle atrophy by inducing the oxidative stress-mediated expression of myostatin and atrogin-1. Sci Rep. 2016; 6: 32084.
- Sato E, Mori T, Mishima E, Suzuki A, Sugawara S, Kurasawa N, et al. Metabolic alterations by indoxyl sulfate in skeletal muscle induce uremic sarcopenia in chronic kidney disease. Sci Rep. 2016; 6: 36618.
- Changchien CY, Lin YH, Cheng YC, Chang HH, Peng YS, Chen Y. Indoxyl sulfate induces myotube atrophy by ROS-ERK and JNK-MAFbx cascades. Chem Biol Interact. 2019; 304: 43-51.
- Thome T, Salyers ZR, Kumar RA, Hahn D, Berru FN, Ferreira LF, et al. Uremic metabolites impair skeletal muscle mitochondrial energetics through disruption of the electron transport system and matrix dehydrogenase activity. Am J Physiol Cell Physiol. 2019; 317: C701-C713.
- Lahiri S, Kim H, Garcia-Perez I, Reza MM, Martin KA, Kundu P, et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci Transl Med. 2019; 11: eaan5662.
- Song J, Wang C, Long D, Li Z, You L, Brand-Saberi B, et al. Dysbacteriosis-induced LPS elevation disturbs the development of muscle progenitor cells by interfering with retinoic acid signaling. FASEB J. 2020; 34: 68376853.
- de Paiva AK, de Oliveira EP, Mancini L, Paoli A, Mota JF. Effects of probiotic supplementation on performance of resistance and aerobic exercises: A systematic review. Nutr Rev. 2023; 81: 153-167.
- Sahin K, Orhan C, Kucuk O, Tuzcu M, Sahin N, Ozercan IH, et al. Effects of magnesium picolinate, zinc picolinate, and selenomethionine co-supplementation on reproductive hormones, and glucose and lipid metabolism-related protein expressions in male rats fed a high-fat diet. Food Chem (Oxf). 2022; 4: 100081.
- Chen LH, Huang SY, Huang KC, Hsu CC, Yang KC, Li LA, et al. Lactobacillus paracasei PS23 decelerated agerelated muscle loss by ensuring mitochondrial function in SAMP8 mice. Aging (Albany NY). 2019; 11: 756-770.
- Katsuki R, Sakata S, Nakao R, Oishi K, Nakamura Y. Lactobacillus curvatus CP2998 prevents dexamethasone-induced muscle atrophy in C2C12 myotubes. J Nutr Sci Vitaminol (Tokyo). 2019; 65: 455458.
- Santamarina AB, Moraes RCM, Nehmi Filho V, Murata GM, de Freitas JA, de Miranda DA, et al. The Symbiotic Effect of a New Nutraceutical with Yeast β-Glucan, Prebiotics, Minerals, and Silybum marianum (Silymarin) for Recovering Metabolic Homeostasis via Pgc-1a, Il-6, and Il-10 Gene Expression in a Type-2 Diabetes Obesity Model. Antioxidants (Basel). 2022; 11: 447.
- Younes S. The efficacy of a 24-hour preoperative pause forSGLT2inhibitors in type II diabetes patients undergoing bariatric surgery to mitigate euglycemic diabetic ketoacidosis. Diabetes Epidemiology and Management. 2024; 14: 100201.
- Younes S. The role of nutrition on the treatment of Covid 19. Human Nutrition & Metabolism. 2024; 36: 200255.
- Younes S. The role of micronutrients on the treatment of diabetes. Human Nutrition & Metabolism. 2024; 35: 200238.
- Younes S. The impact of micronutrients on the sense of taste. Human Nutrition & Metabolism. 2023; 35: 200231.
- Younes Sand, Shbani A. The influence of diabetes on microalbunimuria. Int J Endocrinol Diabetes. 2024; 7: 166.
- Younes S. The impact of b-cell Replication on Diabetes Therapy. Int J Endocrinol Diabetes. 2024; 7: 167.
- Samer Y. Gender Difference in Nutritional Knowledge, Dietary Pattern and Nutritional Status of Undergraduates in Tartous University Syria. Nutri Food Sci Int J. 2024; 13: 555857.
- Samer Y. The Implications of Pyroptosis in Conditions Affecting the Genitourinary Tract. Annals of Urology & Nephrology. 2024; 4.
- Younes S. A Comprehensive Examination of the Nutritional Sufficiency of Vegan Recipes Widely Available in the Market. J. Nutrition. and Food Processing. 2024; 7: 5.