
Research Article
Gerontol Geriatr Res. 2016; 2(2): 1010.
Is there a Role for Procalcitonin in Delirium?
Hamza SA¹, Ali SH¹*, ElMashad NB² and Elsobki HS³
¹Department of Geriatrics and Gerontology, University of Ain Shams, Egypt
²Department of Clinical Pathology, University of Mansoura, Egypt
³Department of Geriatrics, University of Mansoura, Egypt
*Corresponding author: Safaa Hussein Ali, Department of Geriatrics and Gerontology, Faculty of Medicine, University of Ain Shams, Abbassia District, Ramsis Street, Cairo, Egypt
Received: April 05, 2016; Accepted: May 06, 2016; Published: May 09, 2016
Abstract
Background: the pathophysiology of delirium is not fully understood. Inflammation or acute stress responses are less supported Pathophysiological mechanisms.
Objective: To assess the relationship between Procalcitonin (PCT) and delirium among newly admitted delirious elderly patients.
Methods: A case control study was conducted on two groups of elderly hospitalized patients. The case group comprised of 45 elderly patients diagnosed with delirium and compared with 45 elderly patients who are not delirious. Patients were screened, on admission, with “Confusion Assessment Method” CAM, Acute Physiology and Chronic Health Evaluation II (APACHE) score; Erythrocyte Sedimentation Rate (ESR) and Systemic Inflammatory Response (SIR) were assessed.
Results: Delirious patients are older and have higher APACHE score and ESR. Procalcitonin is significantly higher in delirious group in univariant (0.9±0.6 vs. 0.4±0.4ng/mL, P<0.001) and multivariate analysis (OR= 35.59, CI (7.73- 163.76)). Procalcitonin is not affected by presence of inflammation defined by SIR in delirious patients. Prolacitinon had low diagnostic performance as shown by ROC curve (AUC = 0.812, P= <0.001) while APACHE had significantly high diagnostic performance in discrimination of delirium (AUC = 0.877, P= <0.001).
Conclusion: Procalcitonin is rising in delirium independent of age and inflammation.
Keywords: Delirium; Procalcitonin; Elderly; Infection
Abbreviations
APACHE: Acute Physiology and Chronic Health Evaluation II; AUC: Area Under the Curve; CAM: Confusion Assessment Method; CRP: C-Reactive Protein; DSM-IV: Diagnostic and Statistical Manual of Mental Disorders; ESR: Erythrocyte Sedimentation Rate; ICU: Intensive Care Unit; PCT: Procalcitonin; SIR: Systemic Inflammatory Response
Introduction
Delirium is a common and serious condition among the elderly, particularly in hospitalized patients, affecting up to 30% of this patient population [1]. Overall, delirium has been associated with the increase of hospital stay, cognitive decline, functional decline, institutionalization and mortality [2].
The etiology of delirium is usually multifactorial, resulting commonly from a combination of predisposing and precipitating factors. Its pathophysiological mechanisms remain poorly understood, with some evidence for the contribution of neurotransmission disruption, inflammation, or acute stress responses [2].
Procalcitonin (PCT) is an amino acid precursor of calcitonin which under normal circumstances is produced by the thyroid C-cells, Serum concentrations of PCT are normally <0.05 ng/mL but in circumstances of systemic inflammation, particularly bacterial infection, PCT is produced in large quantities by many body tissues. It is detectable within 2-4 hours and peaks within 6-24 hours as opposed to CRP which begins to rise after 12-24 hours and peaks at 48 hours [3].
Although PCT is the pro-hormone for calcitonin, the biologic activities are distinctly different [4]. In the C cells of the thyroid gland and K cells of the lung, elevated serum calcium concentrations or neoplastic changes result in transcription of the PCT gene. Subsequently, ribosomal synthesis of the 116-amino-acid PCT molecule occurs, with subsequent cleavage of amino acids 60 to 91 yielding calcitonin. Calcitonin’s only recognized biologic activity is to lower the serum calcium concentration by inhibiting bone resorption [5].
In the presence of bacterial infection, there is a significant increase in CALC-1 gene expression in parenchymal tissue and in differentiated cell types in the body producing PCT [6]. The rise in PCT levels in bacterial infections is not significantly affected by liver [7] or renal [8] abnormalities, though patients on hemodialysis are an exception in whom elevated PCT levels have been reported in the absence of bacterial infection [9].
Several previous investigations in non-ICU patients established an association between inflammation and delirium, as correlations between proinflammatory cytokine levels and delirium has been found [10]. Delirium is common in systemic inflammatory states which may contribute to delirium pathogenesis through breakdown of the blood–brain barrier, microglial activation, and neuroinflammation [11]. Apart from well-established pro- and anti-inflammatory cytokines [12], PCT plays a role in inflammation, directly associated with delirium [13].
In a prospective study by McGrane et al, inflammatory biomarkers, procalcitonin and CRP (C-reactive protein) were measured in mechanically ventilated patients. Investigators found that higher levels of procalcitonin and CRP were associated with delirium and less coma-free days, implicating inflammation as an important the inflammatory changes within the brain [14]. Our question is: Do Procalcitonin have a role in delirium? Is this role related to inflammation? The aim of this study is to compare between delirious and non-delirious as regard Procalctnin level in a group of elderly hospitalized patients.
Methodology
Subjects
The procedures and rationale for the study were explained to all patients. Because patients had The Confusion Assessment Method, CAM, for delirium at study entry, it was presumed that most were incapable of giving informed written consent. Family caregivers provided a written informed consent using a protocol approved by the local ethics committee of Mansoura university hospitals. A Mansoura university hospital is a tertiary care teaching hospital attached to the faculty of medicine of Mansoura University and its capacity is 255 beds. The hospitals include most of the specialties. Admission rate is about 20:50 cases per day according to available beds and vacant intensive care unit places. This is a case control study. Sample of 90 Egyptian elderly aged sixty years and above divided into 45cases with delirium and 45controls. Subjects have been recruited from Mansoura university hospital over a period of four months.
Extensive demographic and clinical data were also collected at this time including comorbidities. Extensive demographic and clinical data include age, gender, living arrangement, income and financial support, level of education, smoking status, substance abuse, sensory and visual impairment, drug history, surgical history, previous admissions, iatrogenic complications and care giver assessment. Information was collected regarding the co-morbidities. Calculation of Acute Physiology and Chronic Health Evaluation II score (APACHE II): APACHE II score provides a classification of severity of disease. It is calculated from the scores for 12 routine physiologic measurements made during the first 24h after admission. An increasing score (range 0 to 71) was closely correlated with the subsequent risk of hospital death. Mortality risk calculated from APACHE II score [15]. SIR was determined. SIR is defined as a clinical response to a nonspecific insult of either infectious or noninfectious origin. SIR is defined as 2 or more of the following variables:
• Fever of more than 38°C (100.4°F) or less than 36°C (96.8°F
• Heart rate of more than 90 beats per minute
• Respiratory rate of more than 20 breaths per minute or arterial carbon dioxide tension (Pac 2) of less than 32 mm Hg
• Abnormal white blood cell count (>12,000/μL or < 4,000/μL or >10% immature [band] forms) [16]
Inclusion criteria: elderly patient above 60 years old willing to participate in the study.
Exclusion criteria: Patients with the following conditions will be excluded as it leads to elevation of Procalciton: Renal failure, peritoneal dialysis, hemodialysis [9].
-Thyroid cancer [17].
-Acute myocardial infarction [18].
-Acute pancreatitis [19].
-Sever trauma, burns [20] (Figure 1).
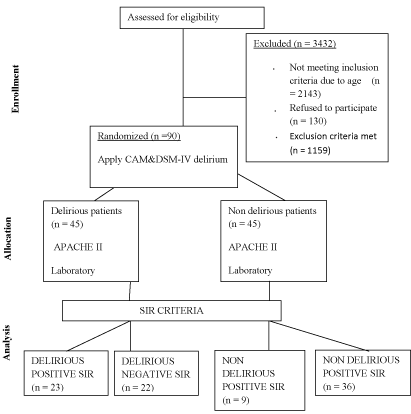
Figure 1: Consort chart of Methodology.
Delirium diagnosis
Diagnostic and Statistical Manual of Mental Disorders, Third Revision (DSM-III-R) criteria for delirium [21] And CAM are used to diagnose delirium [22]. Assessment was done on day of admission and repeated twice daily for three days.
Laboratory tests
Blood sample were withdrawn from all subjects involved in the study on admission and for delirious patients, it is taken when diagnosis was confirmed. It was used for: Complete blood count is performed via Sysmex system (Roch). Arterial blood gases were performed using apl 800 system. ESR was performed using Westergren method. When serum was frozen, it was frozen at -20°C only for 4 months. Human Procalcitonin ELISA (Enzyme-Linked Immunosorbent Assay) kit is an in vitro ELISA for quantitative measurement of human procalcitonin in serum (Human Procalcitonin concentration is pretty low in normal serum/plasma, it may not be detected in this assay). This assay employs an antibody specific for human Procalcitonin coated on a 96-well plate. Standards and Samples are pipette into the wells and Procalcitonin present in a sample is bound to the wells by the immobilized antibody (Ray Bio ® Human Procalcitonin ELISA Kit). Range: 30 pg/ml - 20000 pg/ml; Sensitivity: 30 pg/ml.
Statistical Methods
The collected data were coded, tabulated, and statistically analyzed using IBM SPSS statistics (Statistical Package for Social Sciences) software version 22.0, IBM Corp., Chicago, USA, 2013. Descriptive statistics were done for quantitative data as minimum & maximum of the range as well as mean ±SD (standard deviation) for quantitative parametric data, while it was done for qualitative data as number and percentage. Inferential analyses were done for quantitative variables using independent t-test in cases of two independent groups with parametric data and Chi square test for differences between proportions. Logistic regression model was used to find out independent factors affecting osteoporosis. ROC curve was used to evaluate the performance of different tests differentiate between delirious and non -delirious cases. The level of significance was taken at P value < 0.05 is significant, otherwise is non-significant. Univariate and multivariate logistic regression models were used to predict delirium as a definite diagnosis. Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Values (NPV), and Receiver Operating Characteristic (ROC) curves were also assessed for each factor.
Results
There is no difference between delirious and non-delirious as regard marital status and smoking (P= 0.832&0.619 respectively). Age is significantly higher in delirious group (71.2±7.9 vs. 67.5±8.0 years, p= 0.031). Female represent (60%) of cases and (57.8%) of controls. There was no significant difference between both as regard sex (Table 1). Likewise, APACHE score, its related mortality, SIR and ESR are significantly higher in delirious patients as shown in (Table 1). There is no difference between delirious and non-delirious as regard prevalence of diabetes, hypertension, ischemic heart disease and stroke (P=0.05). Chronic liver diseases were significantly higher in delirious patients (P = 0.05). Procalcitonin is significantly higher in delirious patients in univariate (0.9±0.6 vs. 0.4±0.4ng/mL, P<0.001) and multivariate analysis (OR= 35.59, CI (7.73–163.76)) (Table 2). Procalcitonin was lower in SIR positive patients in delirious patients (Figure 2). Prolacitinonand ESR had low diagnostic performance as shown by ROC curve (AUC = 0.812, P= <0.001 AND AUC = 0.668, P= 0.006) respectively while APACHE had significantly high diagnostic performance in diagnosis of delirium (AUC = 0.877, P= <0.001) (Table 3 & Figure 3).
Variables
Case
(N=45)
Control
(N=45)
P
OR
(95% CI)
Age
(years)
Mean±SD
71.2±7.9
67.5±8.0
^
0.031*
--
Range
60.0–91.0
57.0–92.0
Sex
(N, %)
Male
18 (40.0%)
19 (42.2%)
#
0.830
0.91
(0.39–2.11)
Female
27 (60.0%)
26 (57.8%)
APACHE
Mean±SD
16.4±5.8
8.5±4.0
^
<0.001*
--
Range
6.0–32.0
3.0–22.0
Mortality risk
Mean±SD
25.3±14.9
10.6±6.2
^
<0.001*
--
Range
4.0–73.0
2.0–40.0
ESR
(mm/hr)
Mean±SD
37.5±38.8
8.6±2.9
^
<0.001*
--
Range
4.0–120.0
4.0–16.0
SIR
23 (51.1%)
9 (20.0%)
#
0.002*
4.18
(1.64–10.66)
Procalcitonin(ng/ml)
0.9±0.6
0.4±0.4
<0.001*
0.1–2.7
0.0–1.7
^Independent t-test, #Chi square test, *Significant, OR: Odd Ratio; CI: Confidence Interval
Table 1: Demographic and clinical characteristics among the study groups. Table show that: Age was significantly higher among case group. Total APACHE score, APACHE Mortality risk and ESR were higher among case group.
Variables
β
SE
P
OR (95% CI)
Procalcitonin
3.57
0.78
<0.001*
35.59 (7.73–163.76)
(ng/ml)
β: Regression coefficient; SE: Standard Error; *Significant Odd ratio; CI: Confidence interval
Table 2: Logistic regression for factors increasing the likelihood of delirium. Procalcitoninwas significant factors increases the likelihood of delirium.
Variables
AUC
SE
P
95% CI
APACHE
0.877
0.036
<0.001*
0.806?0.948
Procalciton
0.812
0.045
<0.001*
0.724?0.899
(ng/ml)
ESR
0.668
0.058
0.006*
0.554?0.781
(mm/hr)
AUC: Area Under Curve; SE: Standard Error; *Significant, CI: Confidence Interval
Table 3: ROC curve among the study groups.
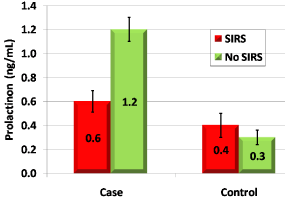
Figure 2: shows that Procalcitonin (ng/ml) is lower in SIR positive patients
in delirious group.
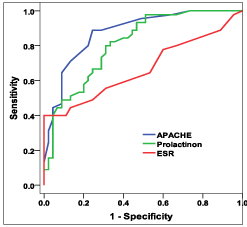
Figure 3: ROC curve among the study groups.
Discussion
The current study shows Significant and independent association between delirium and elevated PCT level. This was confirmed by multiregression analysis which shows that procalcitonin is independently associated with delirium irrespective of other confounders.
This matched with McGrane et al. 2011who found that high baseline inflammatory biomarkers (PCT and ESR) predicted prolonged periods of acute brain dysfunction, implicating inflammation as an important mechanism in the pathophysiology of delirium and coma during critical illness, irrespective of whether patients had sepsis or not [23].
In an exploratory observational study, included ICU patients with or without delirium and with (inflamed) and without (noninflamed) infection/ (SIR) showed that PCT was significantly higher in the delirium group compared with the non-delirium group [24].
In another prospective observational study of adult patients on mechanical ventilation, 24 hours after extubation, PCT was Higher in delirious than non-delirious indicting an association between prolonged inflammation and delirium in the period of mechanical ventilation [25].
On the contrary, a study done by Hirayama and his colleagues, 2015 showed that PCT in ICU admission was not associated with delirium. PCT was almost the same between delirious and nondelirious patients [26]. The difference between that study and the current study may be clear if we know that Hirayamaand his colleagues measured PCT on admission before onset of delirium while in the current study PCT level measured within 24 hours of onset of delirium.
In the current study, a comparison between patients with SIR and patients without ,in delirious patients, revealed that Procalcitonin level is less in patients with SIR than those without in delirious group which indicate that the significant rise of Procalcitonin level in delirious patients was independent of presence of SIR.
Stucker and his colleagues, 2005 ran a study to compare a procalcitonin level on infected and non-infected elderly patients. They found out that PCT may be a useful tool to identify severely ill elderly patients but not to discriminate patients with infections and those without [27].
To explore if Procalcitonin can be used as a helping test to discriminate delirious and none, ROC curve for Procalcitonin is assessed. AUC for ROC was 0.812 (p= <0.001*, 95% CI = 0.724?0.899) which indicate that Procalcitonin had significantly low diagnostic performance in differentiating delirious and none.
This low yield of Procalcionin either to identify delirium or to rise in relation to SIR in elderly may be explained by aging because aging is associated with many functional biological modifications, especially in the immune system. There is now good evidence of a shift from T helper cell 1 and T helper cell 2 productions in older people and also for decreased levels of Tumor Necrosis Factor (TNF) a, IL-1β, and other proinflammatory cytokines in response to acute stress [28]. It has also been demonstrated that TNFβ can stimulate PCT secretion, suggesting that low levels of PCT may be related to these lower cytokine levels [29,30]. In addition, the sensitivity of PCT to detect bacteremia in emergency departments is lower in the elderly population (57%, with a cutoff of 0.5 ng/mL) [31] than in the younger population (92%, with a cutoff of 0.4 ng/mL) [32] suggesting a real effect of senescence on PCT levels.
Conclusion
In elderly patients admitted to an acute care ward with delirium found to have a higher PCT level and irrespective of presence of SIR. Delirious patients have found to be older and are associated with higher mortality in relation to non-delirious patients. Procalcitonin cannot be used to discriminate delirious patients. These results do not allow the systematic use of PCT measurement in acute care wards to detect delirium in older people. Serial PCT levels may be more accurate than a single measurement but a potential use of PCT measurements in severely ill elderly patients could be adopted.
Acknowledgments
We acknowledge the study participants for their gracious help.
References
- Saxena S, Lawley D. Delirium in the elderly: a clinical review. Postgrad Med J. 2009; 85: 405-413.
- Shi Q, Presutti R, Selchen D, Saposnik G. Delirium in acute stroke: a systematic review and meta-analysis. Stroke. 2012; 43: 645-649.
- Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011; 66 Suppl 2: ii33-40.
- Christ-Crain M, Stolz D, Bingisser R, Müller C, Miedinger D, Huber PR, et al. Procalcitonin guidance of antibiotic therapy in community-acquired pneumonia: a randomized trial. Am J Respir Crit Care Med. 2006; 174: 84-93.
- Becker KL, Snider R, Nylen ES. Procalcitonin assay in systemic inflammation, infection, and sepsis: clinical utility and limitations. Crit Care Med. 2008; 36: 941-952.
- Gilbert DN. Use of plasma procalcitonin levels as an adjunct to clinical microbiology. J Clin Microbiol. 2010; 48: 2325-2329.
- Jin M, Khan AI. Procalcitonin: uses in the clinical laboratory for the diagnosis of sepsis. Lab Med. 2010; 41: 173-177.
- Elefsiniotis IS, Skounakis M, Vezali E, Pantazis KD, Petrocheilou A, Pirounaki M, et al. Clinical significance of serum procalcitonin levels in patients with acute or chronic liver disease. Eur J Gastroenterol Hepatol. 2006; 18: 525-530.
- Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol. 2001; 18: 79-87.
- Trimarchi H, Dicugno M, Muryan A, Lombi F, Iturbe L, Raña MS, et al. Pro-calcitonin and inflammation in chronic hemodialysis. Medicina (B Aires). 2013; 73: 411-416.
- Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zimmerli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000; 28: 977-983.
- van Munster BC, Bisschop PH, Zwinderman AH, Korevaar JC, Endert E, Wiersinga WJ, et al. Cortisol, interleukins and S100B in delirium in the elderly. Brain Cogn. 2010; 74: 18-23.
- Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012; 308: 73-81.
- Lesur O, Roussy JF, Chagnon F, Gallo-Payet N, Dumaine R, Sarret P, et al. Proven infection-related sepsis induces a differential stress response early after ICU admission. Crit Care. 2010; 14: R131.
- Aldemir M, Ozen S, Kara IH, Sir A, Baç B. Predisposing factors for delirium in the surgical intensive care unit. Crit Care. 2001; 5: 265-270.
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101: 1644-1655.
- Maruna P, Frasko R, Gürlich R. Plasma procalcitonin in patients with ileus. Relations to other inflammatory parameters. Physiol Res. 2008; 57: 481-486.
- Secmeer G, Devrim I, Kara A, Ceyhan M, Cengiz B, Kutluk T, Buyukpamukcu M. Role of procalcitonin and CRP in differentiating a stable from a deteriorating clinical course in pediatric febrile neutropenia. J Pediatr Hematol Oncol. 2007; 29: 107-111.
- Rau B, Steinbach G, Gansauge F, Mayer JM, Grünert A, Beger HG. The potential role of procalcitonin and interleukin 8 in the prediction of infected necrosis in acute pancreatitis. Gut. 1997; 41: 832-840.
- Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006; 34: 1996-2003.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 3rd ed. Revised (DSM-IIIR). Washington, DC: American Psychiatric Association; 1987.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990; 113: 941-948.
- McGrane S, Girard TD, Thompson JL, Shintani AK, Woodworth A, Ely EW, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011; 15: R78.
- Van den Boogaard M, Kox M, Quinn KL, van Achterberg T, van der Hoeven JG, Schoonhoven L, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and non-inflamed patients. Critical Care. 2011; 15: R297.
- Ogino Y, Kaneda K, Nakahara T, Todani M, Miyauchi T, Fujita M, et al. Systemic inflammation and delirium in the peri-extubation period of mechanical ventilation :an observational prospective study of intensive care unit patients. Bull Yamaguchi Med Sch. 2015; 62: 1-2.
- Hirayama T, Ichiba S, Sato K, Yumoto T, Tsukahara K, Terado M, et al. B-type natriuretic peptide and estimated glomerular filtration rate at ICU admission as a predictor of delirium. Critical Care. 2015; 19: 476.
- Stucker F, Herrmann F, Graf JD, Michel JP, Krause KH, Gavazzi G. Procalcitonin and infection in elderly patients. J Am Geriatr Soc. 2005; 53: 1392-1395.
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004; 39: 687-699.
- Carrol ED, Thomson AP, Hart CA. Procalcitonin as a marker of sepsis. Int J Antimicrob Agents. 2002; 20: 1-9.
- Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta. 2002; 323: 17-29.
- Caterino JM, Scheatzle MD, Forbes ML, D'Antonio JA. Bacteremic elder emergency department patients: procalcitonin and white count. Acad Emerg Med. 2004; 11: 393-396.
- Aalto H, Takala A, Kautiainen H, Repo H. Laboratory markers of systemic inflammation as predictors of bloodstream infection in acutely ill patients admitted to hospital in medical emergency. Eur J Clin Microbiol Infect Dis. 2004; 23: 699-704.