
Special Article - Aging
Gerontol Geriatr Res. 2017; 3(1): 1030.
The Human Perception, Cognition, and Related Epigenetics
Bhadra U¹*, Das P² and Bhadra MP²
¹Centre for Cellular and Molecular Biology, Functional Genomics and Gene Silencing Group, Hyderabad, India
²Centre for Chemical Biology, Indian Institute of Chemical Technology, Hyderabad, India
*Corresponding author: Utpal Bhadra, Centre for Cellular and Molecular Biology, Functional Genomics and Gene Silencing Group, Uppal Road, Hyderabad-500 007, India
Received: May 29, 2017; Accepted: June 28, 2017; Published: July 05, 2017
Abstract
The human brain represents a very complex network of synapses from about 85 billion neurons, which relay important functions of cognition besides other cellular and molecular processes by a multitude of genes and mechanisms. The epigenetic control occurring via changes in the modifications of the nucleosomal components, with no alteration in the DNA sequences is broadly covered, with respect to the alteration of cognitive traits. Normal aging processes occur with the alteration in the activity of the prefrontal cortex and hippocampus and with an increase in synaptic plasticity and neural circuitry. On the contrary, accumulation of various mutations of the DNA by the reactive oxygen species and other mitochondrial mutations accelerate the onset of brain aging with the simultaneous eruption of neurodegenerative diseases and dementia. This manuscript highlights modifications like DNA methylation, hydroxymethylation, histone methylation, acetylation, regulation by BDNF and non-coding RNAs, with details about the epigenetic changes contributing to neurodegeneration.
Keywords: Cognition; Aging; Epigenetic; Neurodegeneration; Non-coding RNAs
Introduction
There are about 86 billion neurons in the human brain, which are from sets of large and small scale synaptic networks [1]. These networks form structures that function as networks for learning and cognition. There are a number of cellular and molecular mechanisms that act as key players in learning and memory. The de novo protein synthesis [2] is one such factor, which is under the control of the expression of a multitude of genes. These genes are in turn orchestrated by a wide array of mechanisms like the epigenetic ones [3] exhibited via modifications of Histone components, nucleosomal components, DNA and RNA molecules. Epigenetic processes are defined as biochemical processes that regulate gene expression without any alteration of the corresponding primary DNA sequence [4].
This review deals with the molecular mediators of epigenomic regulation, which mediate the phenotypic variability in complex behaviors like cognition to be precise. It represents numerous findings on the various histone modifiers particularly, DNA methylation and other epigenetic components. It provides an overview of the status of the field, with respect to both the practical and theory. Thus, it highlights the celebrated the role of epigenetic mechanisms in cognitive processes.
An overview of cognition
Cognition generally refers to the mental processes comprising the gain of knowledge and the ability to comprehend the same. It is a high-level function of the brain which encompasses the activities of thinking, remembering, knowing, judging and problem-solving using features like language, imagination, perception, and planning. According to Neisser (1967), cognition mainly involves:
a) Transforming sensory input
b) Reducing sensory information by means of selective remembrances,
c) Elaborating the information to make it understandable, and
d) storing and recovering information upon requirement,
e) using the information in our actions
Normal age-related memory loss
A normal aged individual show some symptoms of cognitive decline, which can be explained in two heads which are:
Altered activity of the prefrontal cortex and hippocampus: In the normal aging human population, there is a tendency of the reduction in the ability to recall verbal information [5]. Aging normally reduces the working memory, short-term recall, also the speed of information processing, as evident from a longitudinal study performed on subjects aged between 20 to 60 years [6,7]. The age-related memory loss across mammalian species also diminishes spatial memory, as evident in aged humans [8] monkeys, dogs [9], and mice [10]. However, normal aging does not affect the longterm memory of life history, implicit memory, the tendency to unconsciously respond to previously encountered information. Some processes of cognition, like the emotions, improve with aging and emotional stability is attained after the age of 60 due to changing the physiology of the medial prefrontal cortex [11]. Since the activation of the hippocampus is decreased in healthy aged adults, it diminishes the ability to perform tasks which involve recalling of memories or memorizing. Structurally, pathological memory loss is caused due to a loss in the volume of the medial temporal lobes, especially, the entorhinal cortex but in a normal age-related memory loss there is a loss of volume of the pre-frontal cortex [12].
Loss of neural circuits and synaptic plasticity: There is considerable loss of neurons of the neocortex and hippocampus due to aging [13]. An aging hippocampus shows a considerable loss of dendritic branching [14] in contrast to the dendritic branching being variable in the prefrontal cortex [13]. The density of the white matter of the prefrontal cortex and the anterior corpus callosum is also considerably reduced as measured by the diffusion tensor imaging [15]. It is supposed that with age, the frontal cortex create compromised the integrated circuits of the prefrontal cortex, hippocampus, and striatum [12]. Loss of the functional synapse can be responsible for the decline in cognition with age, and density of the synapse of the neurons of the frontal cortex show a significant decline, as evident in humans [16], rats [17] and monkeys [18]. The age-related synapse loss can be clearly seen in the dentate gyrus of the hippocampus, which leads to a loss in the spatial memory [19]. In the rat hippocampus, an impairment of LTP maintenance and induction show severe effects with them becoming susceptible to long-term depression [20]. Synaptic plasticity essential depends upon the regulation of neuronal calcium fluxes and calcium-mediated signaling pathways. Alteration of the calcium homeostasis in brain, therefore, might affect synaptic plasticity. The voltage-activated influx of calcium is increased in the CA1 neurons of the rat hippocampus due to an increase in the L-type calcium channel [21]. The intra neuronal calcium buffering capacity may get impaired due to an altered calcium channel which may increase the free calcium levels in the cytoplasm. The prefrontal cortex of the aging brain shows a reduction in the expression of the mRNA of calbindin1 and signaling proteins like calmodulin 1. These alterations in the expression of the gene thus affect the calcium homeostasis and affect the synaptic plasticity. Reduction in calbindin also makes the neurons more vulnerable to toxic effects such as excitotoxicity, that may lead to neurodegenerative disorders [22,23].
Accelerated Aging Syndromes
Accelerated aging due to faulty DNA repair
Normal age-related memory loss is differentiated from pathological memory loss by the degree of impairment as well as the rate of cognitive decline. Accelerated aging occurs with the inheritance of mutations in DNA repair genes, leading to symptoms called the segmental progeroid syndromes (Figure 1). They show an accelerated onset of a group of human aging phenotypes that include neurodegeneration [24].
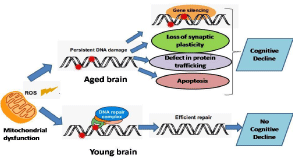
Figure 1: Brain aging and damage to the DNA.
There is oxidative damage to the DNA due to the accumulation of the Reactive Oxygen Species (ROS) because of a mitochondrial dysfunction. In the young adult
brain, this DNA damage is efficiently repaired in the aged brain it keeps on accumulating. In the case of normal aging, this may be caused due to the silencing of
genes involved in synaptic plasticity, mitochondrial function, and protein trafficking. As a result, there is cognitive decline. However, this process may also induce
autophagy as recently reported in type 2 diabetic rats producing Extendin-4 that may provide neuro-protection against cognitive decline.
When genes involved in single or double-strand DNA break repair get mutated it result in cerebellar degenerative syndromes, called ataxia, shown by disorders in movements. When a mutation in the DNA helicases get inherited it results in Werner and Rothmund- Thomson syndromes that accelerate aging without affecting the function of the nervous system. RecQ-like helicases, upon mutation cause syndromes like Xeroderma pigmentosum and Cockayne syndrome with neurodegeneration, mental retardation, and delayed psychomotor development [24]. A progeria-like syndrome is caused due to a mutation in the XPF-ERCC1 endonuclease repairs helixdistorting DNA lesions. When mice were made deficient in ERCC1, it recapitulates progeroid features and exhibits a gene expression profile in the liver compared to that of normal aging mice, which suggest that DNA damage may contribute to the aging process [25].
Mitochondrial dysfunction in aging
Numerous evidence suggests that progressive degeneration and mitochondrial dysfunction accelerate the aging process. Particularly, the aging of postmitotic tissues such as brain and muscle of the cerebral cortex show degeneration in Alzheimer’s disease. The neuronal cells are highly dependent on mitochondrial oxidative phosphorylation to support energy-intensive ion fluxes and axonal transport across long distances in the brain. There are two major sites of mitochondrial damage during aging- the respiratory chain enzymes and mitochondrial DNA [26].
Generation of reactive oxygen species: When the mitochondrial respiratory complexes are inefficient in transporting electrons, it leads to a reduction in ATP synthesis and the superoxide radical generation. Antioxidants like manganese superoxide dismutase, peroxiredoxins, and redox reactions mediated by cytochrome C and cytochrome oxidase are the scavengers for reactive oxygen species. With age, this capacity of ROS scavenging becomes ineffective resulting in the accumulation of local oxidative damage to mitochondrial proteins and DNA. Additionally, hydrogen peroxide is generated by the action of superoxide dismutase on superoxide radicals in mitochondria. Upon diffusing and interaction with transition metals, this hydrogen peroxide is converted by the Fenton reaction to the hydroxyl radical. Elevation in the levels of redox-active iron accumulating in the mitochondria of the brain leads to aging of the brain and several neurodegenerative diseases [27].
Mitochondrial DNA mutations: When the mutation of the mitochondrial DNA gets inherited in the mitochondrial DNA, a decreased mitochondrial base excision repair cause mutations to accumulate in control regions to later impair the transcription and replication of mitochondrial DNA [28] leading to the reduction of the activity of respiratory chain enzymes [29].
The DNA damage gets partially reversed by vitamin E, which suggests a role for ROS generation. Thus, impaired mitochondrial function lead to nuclear DNA damage reduce the expression of nuclear-encoded mitochondrial genes, finally setting up a deleterious feedback loop in the aging brain.
Gene Expression and Animal Models of Aging
The biological basis of cognitive decline with aging is not yet fully understood because, in human subjects, aging is a cumulative effect of numerous age-related disease conditions. Hence, for correctly dissecting the entire biological basis, we need to rely on animal models. As a result, there has been an emergence of useful findings via microarray studies, made in all groups of model organisms like Caenorhabditis elegans, Drosophila, mice, rats, chimpanzees, and humans. To understand the effect of transcription on aging the model of choice is Caenorhabditis elegans [30,31] and Drosophila [32,33], while to study functions specific to the brain, mice [34,35], rats [36], chimpanzees [37] and humans [37,38,39] are used. These studies expose a few aspects about changes occurring due to aging, wherein specific biological processes get altered due to aging more predominately rather than dysregulation of genomewide transcription. Also, there is an incidence of age-related stress response genes and subsequent dysfunction of the mitochondrial genes contributing to increased stress. Point mutations in genes like gene mutations superoxide dismutase and p66shc in Drosophila are found to enhance the lifespan, providing resistance to oxidative stress [29,40,41,42]. In mice, restriction of calories causes an increase in lifespan with increased resistance to oxidative stress in the brain [43].
This means that oxidative stress is a pivotal cause for brain aging.
Only 4% of the frontal cortex genes in the aging brain of human subjects (from 26 to 106 years) have been found to express via transcriptional profiling [38].
Furthermore, expression of age-related genes undergoes alteration apparently only in the middle age only after surpassing the 70 years of age. The genes controlling synaptic functions like mediating memory and learning undergo down-regulation. The glutamate receptor subunits, synaptic vesicle proteins and member carrying out the signal transduction leading to Long Term Potentiation, are some of such members who are the worst affected.
Apparently, the synaptic calcium signaling system gets affected with a reduced expression of calmodulin 1 and 3, CAM kinase IIa´ and IV, calcineurin Ba´, and multiple protein kinase C isoforms. Other age-downregulated genes comprise the ones participating in vesicle-mediated protein transport and mitochondrial function.
The largest categories of genes that undergo up-regulation with age are the stress response genes, including the ones responsible for DNA repair, antioxidant defense, and immune function. Studies in the cortical areas of the brain, particularly the cerebellum and caudate showed that changes with aging mainly comprised a reduction in synaptic function as found in the rat hippocampus, which leads to cognitive impairment [36,37]. Thus, to sum up, it signifies that with aging, the higher order cognitive functions of the mammalian brain get considerably compromised. Studies pertaining to the Alzheimer’s Disease (AD) using microarray studies correlate the expression of the genes to a number of pathological markers like the upregulation of signaling and tumor suppressor genes and downregulation of protein folding, metabolism, and energy-related genes [36]. The results obtained after profiling several affected brain regions showed that VPS35, the retromer trafficking gene correlated with the spatiotemporal pattern as seen in AD [44]. Targeting small interfering RNAs against VPS35 in cell culture studies show that VPS35 probably cause the regulation of the Aβ peptide. A transgenic mouse model expressing AD-linked Amyloid Precursor Protein (APP) and presenilin-1 variants has proven to show specific changes like a reduced cortical Aβ levels and amyloid deposits, when the mice is kept in an environment with upregulated genes for synaptic plasticity, neurogenesis, neuronal survival, and Aβ degradation [45]. Histone deacetylases silence the genes responsible for memory and synapse formation. In the p25/cdk5 mouse model, Inhibitors of Histone deacetylases are found to correct activate the silenced genes, therefore, correcting neurodegeneration [46]. This suggests that there must be genes linking cognition with neuro-degeneration.
Role of Epigenetic Markers in Age-Related Cognition Loss
The methods that have unraveled the underlying epigenetics
Epigenomics involve the identification of features of the DNA sequence that produce epigenomic processes. Such identification is complex, involving multiple steps, with molecular experiments as well as bioinformatics analyses to create large-scale databases.
The International Human Epigenome Consortium is engaged in identifying the cytosine methylation component between various cell types, amongst various cells and tissues. It provides a detailed glimpse of CpG methylation in about 30,000 human genes using 200 cell types [47]. The Human Roadmap Epigenomics project compiles and analyzes a total of 127 reference human epigenomes [48] and raw data, comprising basic DNA sequences, DNA methylation sites, and mRNA levels, after examination in peripheral blood and brain, besides other tissues. A database called Braincloud comprises genomic, epigenomic and transcriptomic data collected from different stages of development, with data for the prefrontal cortex presently annotated.
Studies of epigenetic mechanisms rest on the proper selection of cell types and tissues since epigenetic marks vary between cell types and tissues. Keeping this in mind, to unravel the role of cognitive processes in the brain we need to focus only on the brain tissues. With a complete dearth of human subjects (except for the samples from the post-mortem or surgical tissues), studies such as these are becoming even more difficult. Studies using saliva samples state that DNA methylation correlates to 0. 90 for blood-brain [49,50]. Even if there are various regions of methylome, in various tissues, the largest methylation differences are found within or near genes related to tissue differentiation, particularly neurogenesis and hematopoiesis [51]. Use of such tissues and cells are therefore more replete for studying the epigenetic regulation of cognitive traits. For the first time, a significant study in methylation patterns was observed for the CG-rich promoters across genes [51]. Later studies have made a comparative analysis of methylation pattern between blood and saliva [53] to find out that methylation of DNA showed more resemblance to brain tissues in case of saliva rather than blood [54].
Genome methylation has been earlier postulated as an epigenetic regulator [54,55]. As a result, there have been several methods for studying DNA methylation and histone modification throughout the genome [56].
Restriction Landmark Genomic Scanning (RLGS) is a premier technique for analyzing genome-wide epigenetic patterning [57] using a combination of restriction enzymes, specific to DNA modifications. RLGS cuts the DNA, labeling it with a radioactive isotope for the detection of methylation differences at restriction sites, via two-dimensional gel electrophoresis. The radiation produced by the radioactive labeling exposes the film, in regions where fragments have migrated during electrophoresis. The autoradiographs from the methylated and unmethylated DNA are compared to reveal any changes in the film. Thus, deviations from normal expression are easily detected. It has been found to be exceptionally useful in deciphering hypomethylations and hypermethylation in tumors and also in understanding changes in the methylome during the development of the organism. This technique is tedious but sensitive and has resulted in identifying several imprinted genes [57,58].
Subsequent to this technique, several techniques have been devised, which relies on the enrichment of the genome fraction and polymerase chain reaction for amplification. The inter-methylated sites [59] are amplified using the methylation-sensitive Sma I (Serratia marcescens) restriction enzyme, while the amplification of a methylation target array by cutting with an enzyme sparing the CpG-rich sequences followed by the use of a methylation-sensitive enzyme such as BstU I or Hpa II. Recent technologies overcome all the limitations by using a combination of cell types as the DNA source, to identify the CpGs undergoing methylation from a mixture of methylated/unmethylated patterns [60]. Other technologies utilize the deamination of unmethylated cytosines with sodium bisulfite and the enrichment with targeting antibodies [61,62]. The highthroughput methods to characterize DNA methylation in the central nervous system include the whole-genome bisulfite sequencing, ten– eleven translocation (TET)-assisted bisulfite sequencing, reduced representation bisulfite sequencing, and affinity enrichment based (e.g., MeDIP-Seq).
To analyze the chromatin composition, in terms of Histone composition, chromatin immunoprecipitation (ChIP) is used. It has been found very useful in whole-chromosome oligonucleotide microarray, particularly in identifying transcription factor binding sites [63,64] like the CpG islands [65,66,67] and genomic promoters. Currently, the study of chromatin marks across the entire genome is done by ChIP-Seq, which can sequence the methylome across all the development stages [53]. To study the non-coding RNA, isolation and genotyping or sequencing of small ncRNAs (e.g., microRNAs) as well as long ncRNA is done to understand its role in gene activation, silencing and post-transcriptional gene regulation. The very fact that change in histone modifications and DNA methylation in CNS constitute the formation of memory and manipulating such modifications in experimental animals lead to alteration in memory strongly support the proposition that epigenetic code is deeply rooted in the processes of learning and memory. Memory associated genes like the band, reelin, zif268, PP1, arc, and calcineurin, get epigenetically modified on exposure to an array of responses pertaining to memory and learning. The ultimate challenge that lies ahead for future studies is to determine the comprehensive fashion in which DNA methylation and chromatin remodeling at the level of an individual cell gets regulated and translated with subsequent alteration in the function of the neural circuit during learning and acquiring memory.
Epigenetic markers for cognition
DNA methylation: DNA methylation has been proven to be involved in the execution of multiple processes, such as chromosomal inactivation [68], genomic imprinting [69], silencing of the transposable element and embryonic stem development [70]. DNA methylation occurs when DNA undergoes a covalent alteration via the family, DNA methyltransferases that are involve in the catalysis of the methyl group transfer from S-adenosyl methionine to DNA. Mammals possess five DNMTs - DNMT1, DNMT2, DNMT3a, DNMT3b, and DNMT3L, out of which only three possess the methyltransferase activity namely, the DNMT1, DNMT3a, and DNMT3b. The DNMT1 helps in the sustenance of the methylation after the process of DNA replication [71], such that this epigenetic mark gets inherited in the next generation of the cells. DNA methylation at the fifth position carbon within the cytosine pyrimidine ring is supposing the most well-known epigenetic modifications. The mammalian DNA gets methylated at the promoter regions at the CpG dinucleotides within the genes [72] (Figure 2). In the recent studies, a relatively high amount of non-CpG methylation has been found to occur [73]. A genome-wide study implicated that DNA methylation occurs at different developmental stages in the neurons and glial cells of mice and humans [74]. On the other hand, no-CpG, CH methylation is dominant in the neurons through early childhood and adolescence, gaining more prevalence in the mature neurons. The on-CpG methylation has been proven to be critical in the differentiation of the neurons hence they can effectively predict the subtype-specific gene expression of the neurons compared to their GABAergic interneurons and glutamatergic projection counterparts [75]. Studies that implicated the role of DNA methylation in cognition occurred only in the later years after 2000 [76], where Miller and Sweatt had shown that rats exposed to learning through the Pavlovian fear conditioning paradigm showed an up-regulated expression of de novo mRNA of DNMT3a and DNMT3b in hippocampus. This correlated with the increased DNA methylation of the protein, phosphatase 1 and the genes, reelin - within an hour of the training. The DNA methylation is dynamic in nature and is restored to the normalcy after 24 hrs of the training [76]. DNA methylation has also been found to be related to the expression of the gene, Bdnf, occurring within 2 hr after the training, however, this difference in DNA methylation did not exist after 24 hr [77]. DNMT inhibitors like 5-aza-20-deoxycytidine or zebularine can be used to inhibit DNA-methylation [76] via injection into the hippocampus [77]. It gives an indication that DNA methylation in the adult brain is intricately related with actions constituting new memory via learning and is quite dynamic in nature [46]. The importance of DNA methylation in memory formation also emerges recent studies made on amygdale where it has been demonstrated that the proximal promoter methylation of the human serotonin transporter gene causes a variability of 6.7%-10.4% in the activity of the amygdala in response to threat [78]. Interestingly, the DNA methylation in blood and saliva seemed to be constant and the difference was observed in adolescence and adulthood. This clearly implied that a cross talk existed in the cell culture system and in vivo model where the maternal behavior of the rat induced the epigenetic programming in the offspring’s glucocorticoid gene promoter in the hippocampus [79].

Figure 2: Methylation of the DNA.
(A) The DNA is present in the nucleus in a tightly wrapped form around an octamer of highly basic histone proteins to give rise to chromatin. Various epigenetic
modifications take place at histone tails or directly at DNA. (B) DNA methylation occurs at cytosine bases when a methyl group gets added at the 5' position on the
pyrimidine ring by a DNMT. (c) DNMTs may initiate a de novo DNMTs methylation of the non-methylated cytosines, or maintenance DNMTs may methylate the
hemimethylated DNA at the complementary strand.
DNA hydroxymethylation: Hydroxymethylation of DNA occurs when there is an attachment of a hydroxymethyl group to 5-cytosine. Initially described in the 1970s [80] it was taken into consideration much later [81] when 5hmC’s presence was reported in the cerebellar neurons. The TET proteins were discovered in mammals and their capacity to convert 5mC to 5hmC was then acknowledged [82]. It was proposed that the DNA undergoes methylation by the TET proteins (TET 1-3) via the conversion of 5mC to 5hmC. It would then mediate DNA demethylation by getting converted to 5-formylcytosine and 5-carboxylcytosine [83] Current studies implied that TET1 has a role in regulating neuronal activity [84]. The methyl-CpG binding protein 2 (MeCP2) proteins [85] is one of the protein readers of 5mhC, which is involved in chromatin and transcriptional regulation through binding to 5mC. Hydroxymethylation is pronounced in 25%–40% of modified CG dinucleotides in the frontal cortex and cerebellum [86]. TET proteins are usually expressed in the adult brain [87,88]. Analyzing the whole-genome for the dynamics of 5hmC during the development of the mammalian brain has shown that there are active genomic regions in the adult and fetal mouse brain [74]. The absolute levels of hmC in the fetal brain is much low but in adult patterns, they form the neurons and astrocytes which highlight that hydroxymethylation has a developmental role to play [81]. TET1 has been proved to increase transcriptional flexibility via hydroxymethylation, which transcriptionally regulates cognition [81]. The mRNA levels of TET1 and TET3 apparently change when neuronal activity occurs [84]. Hydroxymethylation studies have been performed in very few model organisms but strongly prove that 5-hydroxymethylcytosine is indeed a result of the DNA demethylation [84,89]. The events of hydroxymethylation help in transcriptional regulation as well as for bringing about long-term changes in the status of the cell and allow heredity of those traits [90,91].
Histone methylation: Methylation of Histone is brought about by the counteracting activity of the players Histone Methyl Transferases and Histone Demethylases. It varies from Acetylation such that in the former, the lysine residues of histones undergo mono-, di-, or trimethylations and that effect of such changes on the transcription can vary based on the position of the lysine residue of the Histone tail that gets altered [92]. Immense numbers of studies show Histone methylation to be involved in neuronal plasticity and cognitive functions. The most predominate ones are involved with H3K4 trimethylation that activates gene promoters.
More than 10 different enzymes are found to catalyze this activity [93]. Fear conditioning activity in rodents has the maximal increase in H3M4me3, where their levels along with the levels of me2 show a correlation with the expression of the glutamate receptors in the human brain [94]. Mutant animals with impaired H3K4 HMTs show a loss of memory [95,96] which may be due to the deregulation of genes related to learning and H3K9 acetylation [96] Histone methylation has a critical role but the direction in which it executes them is very heterogeneous as evident from experiments with HMTs. The very scarce data for demethylases, however, suggest that they have a close relation to cognition [97]. Although much needs to be studied further in this regard.
Histone acetylation: Histone acetylation occurs due to the addition of a negatively charged acetyl group to lysine residues on a histone protein. It reduces the affinity between the positively charged residue and negatively charged DNA [98] and also causes the chromatin to open for translational activities. The acetylation of H2BK5, H3K14, H4K5, and H4K12 has been found to be effective in cognition [99,100]. Histone acetylation is executed by Histone Acetyltransferases (HATs) that catalytically adds acetyl group, while HDACs counteracts the activity by removing the acetyl group. The HATs and HDACs have subfamilies like the Gcn5-related N-Acetyltransferases (GNATs), the MYST (includes the MOZ, Ybf2, Sas2, and Tip60), the p300/CREB-binding protein histones (p300/CBP), the zinc-dependent Classes I, II, and IV, and the NADdependent Class III [101].
In the 1970s, studies showed that histone acetylation gets altered during memory consolidation in rats. Histone acetylation gets intensified after a neuronal activity, which promotes changes in the expression of the genes causing long-term synaptic plasticity and memory [102]. Initially, histone acetylation has been found to get initiated as a result of a number neuronal activity, like the neurotransmitter pathway stimulation by agonists specific to the receptor [103], neuronal depolarization mediated by potassium chloride [104], and, the mitogen-activated protein kinase pathway activation, via direct [105] or indirect cross-talk with other types of histone modification [106]. Histone acetylation causes the promotion of a long-term synaptic plasticity. Histone Acetylation has been found to be related to long-term facilitation which increases the histone acetylation in the genes related to LTF and in genes responsible for long-term depression, histone acetylation is reduced. When H3 and H4 acetylation gets intensified at the promoter regions of the rodent gene, Bdnf, [105] it induces long-term potentiation [107].
Histone acetylation apparently is also associated with the formation of memory, as is seen in the case of Bdnf whose expression increases with Acetylation [108]. Histone acetylation excites positive reciprocal processes, causing the modulation of gene expression, and subsequent modification of the histones [102].
About 11 of the mammalian HDACs called zinc-dependent HDACs, need Zn2þ ion as a cofactor [109,110]. All HDAC genes are usually expressed in an adult brain [111] and a related to cognition. But very less is known about the role of NAD-dependent Class III HDACs, known as sirtuins in cognition. The zinc-dependent HDACs include Class I (HDAC1–HDAC3 and HDAC8), Class IIa (HDAC4, HDAC5, HDAC7, and HDAC9), Class IIb (HDAC6 and HDAC10), and Class IV (HDAC11). For example, Class I HDACs 1–3 are negatively associated with learning and memory [108,112,113]. HDAC1 has been found to be involved in memory extinction [114] and HDAC2 and HDAC3 in memory constraints [115]. HDAC2 has an evident decrease in expression of Histone Acetylation, which is seen in Alzheimer disease and aging [116]. Limited evidences suggest that Class IIa HDACs 4, 5, 7, and 9 are involved in cognition [102], with HDAC4 and HDAC5 [49,115,117] being more prevalent than HDAC7 and HDAC9. A downregulation of HDAC4 in adult mice brains is associated with memory formation and plasticity impairment [118].
The Haploin sufficiency of HDAC4 is also responsible for a disabled intellect. On the contrary, there are about 18 mammalian HATs grouped in families of the GNAT, the MYST, and the p300/ CBP; and many other HATs that cannot be grouped. There are various nonspecific histone deacetylase inhibitors, like trichostatin A, suberoylanilide hydroxamic acid, sodium butyrate, phenylbutyrate, and valproic acid that have been studied on understanding more about learning and memory in mice and rats. They have given conclusions that if treatments are given using these inhibitors; they can help to cure the different cognitive deficits like traumatic brain injury, neurodegeneration and targeted mutations [81]. All such evidence clearly cites that they are very much essential players in the modulation of learning and memory.
BDNF gene regulation: BDNF is a small secreted protein that belongs to the neurotrophin family of growth factors which helps in establishing LTP, helping in sustaining neuroplasticity [119,120,121]. BNDF is well-known for its role in general neural development. There is an in-vitro connection between neuronal depolarization and hypomethylation within the transcriptional regulatory region of the BDNF gene with subsequently increases the BDNF mRNA expression [122]. This acted as a foundation stone for dissecting the role of DNA methylation as a potential mediator of transcriptional regulation mediated by the neuronal activity within the CNS. Thus, was established the hypothesis of epigenetic dynamic regulation which also stated that transcriptional control by BDNF was essential for synaptic plasticity.
The evidence for the epigenetic regulation of BDNF was exposed via studies made on the rodent subjects [123], where it was demonstrated that MeCP2 represses the transcription of Bdnf gene [124,125]. In rodents and humans, the BDNF gene bears multiple promoters that orchestrate transcription orchestrating transcription. The fourth promoter of Bdnf, responsible for cognitive processes [92], bears specific sites of binding for CREB, which also play a part in the remodelling of chromatin [65]. The phosphorylation of the fourth promoter increases the transcription of the Bdnf gene and dissociates the MeCP2 (Figure 3). The HDAC1joins with Sin3a, causing the Bdnf gene to remain in a repressed state of transcriptional activity [122]. The DNA demethylation of Bdnf promoter IX due to neuronal activity is reported to increase the neurogenesis in the hippocampus [126]. BDNF is a well-known neuroplasticity mediating agent which has been proven to cause the stimulation of neurons bearing them. This, in turn, leads to the nitrosylation of HDAC2 on cysteine (C) 262 and C 274, histone hyperacetylation, and a consecutively an increase in neurotrophin-dependent gene expression [127]. The BDNF gene expression gets intensified when neuronal activity drives the calciumdependent derepression by MeCP2. BDNF gets negatively regulated by HDAC2 which proves that a positive-feedback loop is formed between HDAC2 and BDNF due a sudden increase in the neuronal activity. Such activities lead to the expression of the genes due to histone acetylation. This can, therefore, also lead to a change in the synaptic strength and thus affect learning.
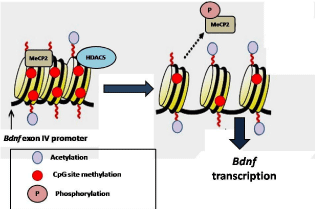
Figure 3: Epigenetic regulation of Bdnf gene for cognition in rodents.
BDNF performs epigenetic regulation of cognition in rodents. MeCP2 acts as the transcriptional repressor of the Bdnf gene. The BDNF gene promoter IV bears
specific sites for CREB binding that remodels the chromatin. The phosphorylation of the fourth promoter leads to the dissociation of the MeCP2 and increase the
transcription of the Bdnf gene. Additionally, HDAC5 is also involved in the regulation of the Bdnf gene.
Non-coding RNAs: Non-coding RNAs represent two classes of RNAs, one below the length of 200 nucleotides referred to as small and above 200 bases, referred to as long. The brain houses an array of numerous ncRNAs varying in diversity and prominence [128].
The miRNAs are most extensively studied group of ncRNAs, which are 12-22 at long that catalyze gene silencing by binding to a target messenger RNA. This RNA, in turn, stimulates either its degradation or the inhibition of protein translation and thus, the protein homeostasis is regulated [129]. There is a doubt whether ncRNAs should be viewed as one of the epigenetic players, but there are many pieces of evidence to support this proposition [130]. In certain neurons, specific miRNAs regulate a number of target genes via controlling their protein secretion, like in the case of miR-134 which has been found to negatively regulate the size of dendritic spines. The miR-134 inhibits the mRNA of Limk1, a protein kinase that controls spine development via processes like mRNA trafficking. miR-134 is also believed to regulate neuronal plasticity. miR-134, when over expressed in the hippocampus C1 area, leads to a significant long-term memory impairment using the contextual fear conditioning model. On the other hand, a reduction in miR-134 invivo leads to an increased memory function.
miRNAs are proven to be important regulators of synaptic plasticity [131,132,133] and memory consolidation, as seen in regulation of the cyclic- AMP-responsive element-binding protein (CREB) in serotonin-induced synaptic plasticity [134,135]. The maturation of miRNA requires Dicer, so miRNA may act as a molecular brake to the formation of memory and may be essential for maintaining neuronal homeostasis.
Long noncoding RNAs (lncRNAs) have an important role in neural development and functioning. They have been found capable of inducing hypomethylation in gene promoters, to regulate the expression of the gene [136]. Recent studies have shown that BC048612, a long ncRNA, which is co-regulated with miR-203 control the expression of neuronal growth regulator 1 (NEGR1) cell adhesion protein in neurons [137]. The expression of lncRNA increases NEGR1 during early maturation of the neurons, exerting the regulatory control being “passed to” the miRNA during later stages to fine-tune the expression of NEGR1. Therefore, ncRNAs present a very promising molecule for dissecting the brain organization of gene expression pertaining to cognition.
Epigenetic players in neuro-degeneration: The clinical studies pertaining to neurodegeneration provide ample evidence about dissecting the role of epigenetic mechanisms in cognition and learning. Developmental disorders include the Rubinstein-Taybi Syndrome (RTS), Rett Syndrome (RT), and Kabuki Syndrome (KS) and neurodegenerative disorders, include the Huntington disease (HD) and Alzheimer’s disease AD [138].
RTS, an autosomal dominant condition [139] caused by mutations in the histone acetyltransferase, CBP/KAT3A or EP300 [140]. It affects 1 in 125,000 to 1 in 720,000 births. It is characterized by anatomical abnormalities and the subjects have severe intellectual disability.
RTT is an X-linked dominant developmental disorder caused by mutations in MeCP2 [141,142,143]. It shows a symptom of developmental regression, such that development progresses normally until 6-18 months, but suffers severe developmental stagnation with microcephaly, hypotonia, weight loss, and severe mental retardation [144,145,146].
KS is a developmental disorder occurring in almost 1 in 32,000 newborns [147] due to mutations in the gene, lysine-specific methyltransferase 2D, MLL2or KMT2D on the chromosome 12q13. It is characterized by the presence of pale, chalky facial skin, bodily dysmorphology, developmental delay, intellectual disability, and microcephaly, hypotonia, and nystagmus or strabismus.
HD is an autosomal dominant, progressive, neurodegenerative disorder. The affected individuals have phenotypes like chorea, lack of coordination, cognitive decline, dystonia and behavioral difficulties. There is a progressive, selective neural cell loss and atrophy in the caudate and putamen [148].
Alzheimer’s disease, a neurodegenerative disorder, represents progressive dementia in the elderly individuals. It is a characterized by the formation of amyloid-beta plaques, neurofibrillary tangles, and severe neuronal loss in the brain, leading to dementia [138]. It may have a familial onset of about 5%, which occur early or may have late onset form comprising about 95%, occurring due to a genetic and environmental risk factors [149]. Recent studies have shown that multiple epigenetic mechanisms like histone acetylation, DNA methylation, and ncRNAs have [150] biological relevance to the manifestation of the latter forms of AD and can help in designing novel therapeutic for AD.
Epigenetic control of cognition by autophagy: Autophagy involves the self-digestion strategy for maintaining the cellular homeostasis and viability when subjected to nutrient and energyrelated stresses [151,150,152,153]. A dysregulation of autophagy is often the root of ailments such as cancer and neurodegenerative diseases [154]. Previous works have suggested that autophagy is but a cytoplasmic event but recent studies unfolded that the process of autophagy is under the stringent epigenetic and transcriptional control occurring in the nucleus of the cell [155]. Studies point out that histone modifications by CARM1 H3R17 methyltransferase [156] G9a H3K9 methyltransferase [157], EZH2 H3K27 methyltransferase [158], SIRT1 H4K16 deacetylase and hMOF H4K16 acetyltransferase [159] are critical regulators of autophagy.
Any damage to the intracellular machinery requires degradation by the ubiquitin-proteasome system or the lysosomal/autophagic pathway [152] to prevent its accumulation. The conventional method of autophagy is a highly non-selective intracellular pathway to respond to stress. The deregulated autophagy in the case of Type 2 Diabetes or Alzheimer’s Disease is the result of the accumulation of neuropathological markers like the neurofibrillary tangles (NFTs) and amyloid plaques in Alzheimer’s disease [160,161,162]. The accumulation may also be a result of aberrant clearing of dysfunctional mitochondria, endoplasmic reticulum, protein aggregates and lysosomal defects affecting the chaperonemediated autophagy [162]. The induction of autophagy requires a number of conserved metabolites such as mTOR and 5' adenosine Monophosphate-Activated Protein Kinase (AMPK), that are also active links to the insulin/IGF-1 intracellular signaling [163]. Autophagy is found to be repressed in situations of a decreased AMPK to normal aging, Type 2 diabetes, and Alzheimer’s disease. As the result the protein clearance pathway gets blocked causing accumulation of misfolded or aggregated proteins in pancreatic β cells in case of Type 2 diabetes or in neurons in case of Alzheimer’s disease [164,165,166] cause the progression of the respective diseases. This makes Type 2 diabetes central to neurodegenerative disorders due to increased insulin resistance in the brain, glucose dysmetabolism, autophagic alterations, neuronal death and cognitive impairments [167,168,169]. Experiments have shown that if brain cortical GLP-1 level and insulin level in middle-aged Type-2 diabetic rats are maintained via subcutaneous doses of Exendin-4 to activate the PKA and PI3K/Akt signalling, it results in autophagy, which will help in the clearance of misfolded/ unfolded proteins and damaged organelles to give protection against cortical injury to the brain due to Type-2 diabetes. It can cure long-term complications pertaining to cognitive impairment and neurodegeneration due to Alzheimer’s disease. Thus, it clarifies that autophagy acts a housekeeper to clear off the accumulated dysfunctional proteins, organelles and therefore protects the brain cortical tissues and neurons from damage and delays the onset of aging.
Conclusion
In spite of the enormous diversity of the data and findings achieved so far, the crucial evidence related to the importance of the development of epigenetic mechanisms, its manifestation in subjects and degeneration of cognitive functions is still unclear. Epigenetic regulations of cognition, at the macroscopic level, are significant. There is all evidence obtained mainly from studies performed on animal models with very less data on human subjects. All the animal model studies utilize the tissues of the brain as a substance of cognition to understand the mechanism of regulation which is not possible with humans. Certain mechanisms are performed on the post-mortem tissues, however, to understand mechanisms having a time scale; it remains uncaptured in such tissues. It also becomes impossible to establish the developmental patterns in the functioning of the epigenome, in mapping the alterations in the neural circuits to support behaviors related to cognitive development and processing. The scientific community must become aware of the methodological, statistical, and interpretational issues that are associated with epigenome- wide association studies approaches. But still much is left to get revealed in this field.
Acknowledgement
We have dedicated this paper to the memory of Dr. Malaya Kr. Pal, a renowned doctor of Kolkata, who was the authors’ inspiration in Science.
Author Contributions
UB and MPB have conceived the idea, PD has written the initial draft and finalized with UB and MPB.
Funding
This work was supported by the CSIR network project to UB (BSC0121, BSC 0108) and DBT grant and CSIR SMILE project to MPB. PD was supported by the ICMR JRF fellowship award (GAP 0502).
References
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, et al. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. Journal of Comparative Neurology. 2009: 513: 532-541.
- Flexner JB, Flexner LB, Stellar E. Memory in mice as affected by intracerebral puromycin. Science. 1963; 141: 57-59.
- Allis CD, Jenuwein T, Reinberg D, Caparros ML. Epigenetics. 2007.
- Nikolova YS, Hariri AR. Can we observe epigenetic effects on human brain function?. Trends in cognitive sciences. 2015; 19: 366-373.
- Albert M, Duffy FH, Naeser M. Nonlinear changes in cognition with age and their neurophysiologic correlates. Canadian Journal of Experimental Psychology. 1987; 41: 141-157.
- Craik FI, Moscovitch M, McDowd JM. Contributions of surface and conceptual information to performance on implicit and explicit memory tasks. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994; 20: 864-875.
- Zelinski EM, Burnight KP. Sixteen-year longitudinal and time lag changes in memory and cognition in older adults. Psychology and Aging. 1997; 12: 503-513.
- Montgomery EB, Koller WC, LaMantia TJ, Newman MC, Swanson-Hyland E, Kaszniak AW, et al. Early detection of probable idiopathic Parkinson’s disease: I. Development of a diagnostic test battery. Movement disorders. 2000; 15: 467-473.
- Lai ZC, Moss MB, Killiany RJ, Rosene DL, Herndon JG. Executive system dysfunction in the aged monkey: spatial and object reversal learning. Neurobiology of Aging. 1995; 16: 947-954.
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, et al. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proceedings of the National academy of sciences. 1999; 96: 5280-5285.
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years?: neural basis of improving emotional stability over age. Journal of Neuroscience. 2006; 26: 6422-6430.
- Hedden T, Gabrieli JD. Insights into the aging mind: a view from cognitive neuroscience. Nature reviews Neuroscience. 2004; 5: 87-96.
- Burke SN, CA Barnes. Neural plasticity in the aging brain. Nature reviews Neuroscience. 2006; 7: 30-40.
- Buell S. Dendritic growth in normal human aging and apparent lack of growth in senile dementia: A computer-assisted quantitative study. Ph. D. Thesis (10th editionn.) University of Rochester, New York. 1979.
- Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. American Psychological Association. 2000.
- Liu X, Erikson C, Brun A. Cortical synaptic changes and gliosis in normal aging, Alzheimer’s disease and frontal lobe degeneration. Dementia and Geriatric Cognitive Disorders. 1996; 7: 128-134.
- Geinisman Y, De Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: a morphological substrate of memory deficit in aged rats. Proceedings of the National academy of sciences. 1986; 83: 3027-3031.
- Bourgeois JP, Rakic P. Synaptogenesis in the occipital cortex of macaque monkey devoid of retinal input from early embryonic stages. European Journal of Neuroscience. 1996; 8: 942-950.
- Landfield PW, Lynch G. Impaired monosynaptic potentiation in in vitro hippocampal slices from aged, memory-deficient rats. Journal of Gerontology. 1977; 32: 523-533.
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of longterm depression and long-term potentiation reversal during aging. Journal of Neuroscience. 1996; 16: 5382-5392.
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996; 272: 1017-1020.
- Mattson MP, Rychlik B, Chu C, Christakost S. Evidence for calcium-reducing and excitoprotective roles for the calcium-binding protein calbindin-1328k in cultured hippocampal neurons. Neuron. 1991; 6: 41-51.
- Geula C, Bu J, Nagykery N, Scinto LF, Chan J, Joseph J, et al. Loss of calbindin-D28k from aging human cholinergic basal forebrain: Relation to neuronal loss. Journal of Comparative Neurology. 2003. 455: 249-259.
- Hasty P, Campisi J, Hoeijmakers J, Van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse?. Science. 2003; 299: 1355-1359.
- Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, et al. A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature. 2006; 444: 1038-1043.
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005; 39: 359-407.
- Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR. Iron, brain aging and neurodegenerative disorders. Nature reviews Neuroscience. 2004; 5: 863-873.
- Coskun PE, Beal MF, Wallace DC. Alzheimer’s brains harbor somatic mtDNA control-region mutations that suppress mitochondrial transcription and replication. Proceedings of the National Academy of Sciences of the United States of America. 2004; 101: 10726-10731.
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998; 282: 943-946.
- Lund J, Tedesco P, Duke K, Wang J, Kim SK, Johnson TE. Transcriptional profile of aging in C. elegans. Current Biology. 2002; 12: 1566-1573.
- McCarroll SA, Murphy CT, Zou S, Pletcher SD, Chin CS, Jan YN, et al. Comparing genomic expression patterns across species identifies shared transcriptional profile in aging. Nature Genetics. 2004; 36: 197-204.
- Zou S, Meadows S, Sharp L, Jan LY, Jan YN. Genome-wide study of aging and oxidative stress response in Drosophila melanogaster. Proceedings of the National academy of sciences. 2000; 97: 13726-13731.
- Pletcher SD, Macdonald SJ, Marguerie R, Certa U, Stearns SC, Goldstein DB, et al. Genome-wide transcript profiles in aging and calorically restricted Drosophila melanogaster. Current Biology. 2002; 12: 712-723.
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the aging brain in mice. Nature genetics. 2000; 25: 294-297.
- Jiang CH, Tsien JZ, Schultz PG, Hu Y. The effects of aging on gene expression in the hypothalamus and cortex of mice. Proceedings of the national academy of sciences. 2001; 98: 1930-1934.
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. Journal of Neuroscience. 2003; 23: 3807-3819.
- Fraser HB, Khaitovich P, Plotkin JB, Pääbo S, Eisen MB. Aging and gene expression in the primate brain. PLoS Biol. 2005; 3: e274.
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, et al. Gene regulation and DNA damage in the ageing human brain. Nature. 2004; 429: 883-891.
- Erraji-Benchekroun L, Underwood MD, Arango V, Galfalvy H, Pavlidis P, Smyrniotopoulos P, et al. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biological Psychiatry. 2005; 57: 549-558.
- Orr WC, RS Sohal. Extension of life-span by over expression of superoxide dismutase and catalase in Drosophila melanogaster. Science. 1994; 263: 1128-1130.
- Parkes TL, Elia AJ, Dickinson D, Hilliker AJ, Phillips JP, Boulianne GL. Extension of Drosophila lifespan by overexpression of human SOD1 in motorneurons. Nature Genetics. 1998; 19: 171-174.
- Migliaccio E, Giorgio M, Mele S, Pelicci G, Reboldi P, Pandolfi PP, et al. The p66shc adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999; 402: 309-313.
- Hyun DH, Emerson SS, Jo DG, Mattson MP, de Cabo R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proceedings of the national academy of sciences. 2006; 103: 19908-19912.
- Small SA, Kent K, Pierce A, Leung C, Kang MS, Okada H, et al. Modelguided microarray implicates the retromer complex in Alzheimer’s disease. Annals of neurology. 2005; 58: 909-919.
- Lazarov O, Robinson J, Tang YP, Hairston IS, Korade-Mirnics Z, Lee VMY, et al. Environmental enrichment reduces Aβ levels and amyloid deposition in transgenic mice. Cell. 2005; 120: 701-713.
- Fischer A, Sananbenesi F, Wang X, Dobbin M, Tsai LH. Recovery of learning and memory is associated with chromatin remodeling. Nature. 2007; 447: 178-182.
- Bradbury J. Human epigenome project–up and running. PLoS Biol. 2003; 1: e82.
- Consortium RE, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. 2015; 518: 317- 330.
- Horvath S, Zhang Y, Langfelder P, Kahn RS, Boks MP, van Eijk K, et al. Aging effects on DNA methylation modules in human brain and blood tissue. Genome Biology. 2012; 13: R97.
- Tylee DS, Kawaguchi DM, Glatt SJ. On the outside, looking in: A review and evaluation of the comparability of blood and brain “omes”. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2013; 162: 595-603.
- Davies MN, Volta M, Pidsley R, Lunnon K, Dixit A, Lovestone S, et al. Functional annotation of the human brain methylome identifies tissuespecific epigenetic variation across brain and blood. Genome Biology. 2012; 13: R43.
- Thompson TM, Sharfi D, Lee M, Yrigollen CM, Naumova OY, Grigorenko EL. Comparison of whole-genome DNA methylation patterns in whole blood, saliva, and lymphoblastoid cell lines. Behavior Genetics. 2013; 43: 168-176.
- Smith AK, Kilaru V, Klengel T, Mercer KB, Bradley B, Conneely KN, et al. DNA extracted from saliva for methylation studies of psychiatric traits: evidence tissue specificity and relatedness to the brain. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2015; 168: 36-44.
- Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenetic and Genome Research. 1975; 14: 9-25.
- Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Cold Spring Harbor Monograph Series. 1996; 32: 639-645.
- Fazzari MJ, Greally JM. Epigenomics: beyond CpG islands. Nature Reviews Genetics. 2004; 5: 446-455.
- Shibata H, Hirotsune S, Okazaki Y, Komatsubara H, Muramatsu M, Takagi N, et al. Genetic mapping and systematic screening of mouse endogenously imprinted loci detected with restriction landmark genome scanning method (RLGS). Mammalian Genome. 1994; 5: 797-800.
- Plass C, Shibata H, Kalcheva I, Mullins L, Kotelevtseva N, Mullins J, et al. Identification of Grf1 on mouse chromosome 9 as an imprinted gene by RLGS–M. Nature Genetics. 1996; 14: 106-109.
- Frigola J, Ribas M, Risques RA, Peinado MA. Methylome profiling of cancer cells by amplification of inter-methylated sites (AIMS). Nucleic acids research. 2002; 30: e28.
- Novik K, Nimmrich I, Genc B, Maier S, Piepenbrock C, Olek A, et al. Epigenomics: genome-wide study of methylation phenomena. Current issues in molecular biology. 2002; 4: 111-128.
- Laird PW. Principles and challenges of genome-wide DNA methylation analysis. Nature Reviews Genetics. 2010; 11: 191-203.
- Heyward FD, Sweatt JD. DNA Methylation in Memory Formation Emerging Insights. The Neuroscientist. 2015; 21: 475-489.
- Martone R, Euskirchen G, Bertone P, Hartman S, Royce TE, Luscombe NM, et al. Distribution of NF-?B-binding sites across human chromosome 22. Proceedings of the national academy of sciences. 2003; 100: 12247-12252.
- Cawley S, Bekiranov S, Ng HH, Kapranov P, Sekinger EA, Kampa D, et al. Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell. 2004; 116: 499-509.
- Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002; 33: 383-395.
- Weinmann AS, Yan PS, Oberley MJ, Huang THM, Farnham PJ. Isolating human transcription factor targets by coupling chromatin immunoprecipitation and CpG island microarray analysis. Genes & development. 2002; 16: 235- 244.
- Wells J, Yan PS, Cechvala M, Huang T, Farnham PJ. Identification of novel pRb binding sites using CpG microarrays suggests that E2F recruits pRb to specific genomic sites during S phase. Oncogene. 2003; 22: 1445-1460.
- Pfeifer G, Steigerwald S, Hansen RS, Gartler S, Riggs A. Polymerase chain reaction aided genomic sequencing of an X chromosome-linked CpG island: methylation patterns suggest clonal inheritance, CpG site autonomy, and an explanation of activity state stability. Proceedings of the National Academy of sciences. 1990; 87: 8252-8256.
- Bartolomei MS, Webber AL, Brunkow ME, Tilghman SM. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes & development. 1993; 7: 1663-1673.
- Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nature genetics. 2003; 33: 245-254.
- Bird A. DNA methylation patterns and epigenetic memory. Genes & development. 2002; 16: 6-21.
- Bird AP. DNA methylation and the frequency of CpG in animal DNA. Nucleic acids research. 1980; 8: 1499-1504.
- Portela A, Esteller M. Epigenetic modifications and human disease. Nature Biotechnology. 2010; 28: 1057-1068.
- Lister R, Mukamel EA, Nery JR, Urich M, Puddifoot CA, Johnson ND, et al. Global epigenomic reconfiguration during mammalian brain development. Science. 2013; 341: 1237905.
- Kozlenkov A, Wang M, Roussos P, Rudchenko S, Barbu M, Bibikova M, et al. Substantial DNA methylation differences between two major neuronal subtypes in human brain. Nucleic acids research. 2016; 44: 2593-2612.
- Miller CA, Sweatt JD. Covalent modification of DNA regulates memory formation. Neuron. 2007; 53: 857-869.
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. Journal of Neuroscience. 2008; 28: 10576-10586.
- Nikolova YS, Koenen KC, Galea S, Wang CM, Seney ML, Sibille E, et al. Beyond genotype: serotonin transporter epigenetic modification predicts human brain function. Nature Neuroscience. 2014; 17: 1153-1155.
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nature Neuroscience. 2004; 7: 847-854.
- Penn N, Suwalski R, O’riley C, Bojanowski K, Yura R. The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic acid. Biochemical Journal. 1972; 126: 781-790.
- Rudenko A, Tsai LH. Epigenetic modifications in the nervous system and their impact upon cognitive impairments. Neuropharmacology. 2014; 80: 70- 82.
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009; 324: 930-935.
- Pastor WA, Aravind L, Rao A. TETonic shift: biological roles of TET proteins in DNA demethylation and transcription. Nature reviews Molecular cell biology. 2013; 14: 341-356.
- Kaas GA, Zhong C, Eason DE, Ross DL, Vachhani RV, Ming Gl, et al. TET1 controls CNS 5-methylcytosine hydroxylation, active DNA demethylation, gene transcription, and memory formation. Neuron. 2013; 79: 1086-1093.
- Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, et al. “Dynamic readers for 5-(hydroxy) methylcytosine and its oxidized derivatives.” Cell. 2013; 152: 1146-1159.
- Kinde B, Gabel HW, Gilbert CS, Griffith EC, Greenberg ME. “Reading the unique DNA methylation landscape of the brain: non-CpG methylation, hydroxymethylation, and MeCP2.” Proceedings of the national academy of sciences. 2015; 112: 6800-6806.
- Kriaucionis S, Heintz N. “The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain.” Science. 2009; 324: 929-930.
- Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, et al. “5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging.” Nature Neuroscience. 2011; 14: 1607-1616.
- Rudenko A, Dawlaty MM, Seo J, Cheng AW, Meng J, Le T, et al. “Tet1 is critical for neuronal activity-regulated gene expression and memory extinction.” Neuron. 2013; 79: 1109-1122.
- Guan JS, Xie H, Ding X. “The role of epigenetic regulation in learning and memory.” Experimental Neurology. 2015; 268: 30-36.
- Shukla A, Sehgal M, Singh TR. “Hydroxymethylation and its potential implication in DNA repair system: A review and future perspectives.” Gene. 2015; 564: 109-118.
- Lipsky RH. “Epigenetic mechanisms regulating learning and long-term memory.” International Journal of Developmental Neuroscience. 2013; 31: 353-358.
- Badeaux AI, Shi Y. “Emerging roles for chromatin as a signal integration and storage platform.” Nature reviews Molecular cell biology. 2013; 14: 211-224.
- Stadler F, Kolb G, Rubusch L, Baker SP, Jones EG, Akbarian S. “Histone methylation at gene promoters is associated with developmental regulation and region-specific expression of ionotropic and metabotropic glutamate receptors in human brain.” Journal of neurochemistry. 2005; 94: 324-336.
- Gupta RA, Shah N, Wang KC, Kim J, Horlings HM, Wong DJ, et al. “Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis.” Nature. 2010; 464: 1071-1076.
- Kerimoglu C, Agis-Balboa RC, Kranz A, Stilling R, Bahari-Javan S, Benito- Garagorri E, et al. “Histone-methyltransferase MLL2 (KMT2B) is required for memory formation in mice.” Journal of Neuroscience. 2013; 33: 3452-3464.
- Rujirabanjerd S, Nelson J, Tarpey PS, Hackett A, Edkins S, Raymond FL, et al. “Identification and characterization of two novel JARID1C mutations: suggestion of an emerging genotype-phenotype correlation.” European Journal of Human Genetics. 2012; 20: 1010.
- Brownell JE, Allis CD. “Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation.” Current opinion in genetics & development. 1996; 6: 176-184.
- Gräff J, Mansuy IM. “Epigenetic codes in cognition and behavior.” Behavioural brain research. 2008; 192: 70-87.
- Puckett RE, Lubin FD. “Epigenetic mechanisms in experience-driven memory formation and behavior.” Epigenomics. 2011; 3: 649-664.
- Haberland M, Montgomery RL, Olson EN. “The many roles of histone deacetylases in development and physiology: implications for disease and therapy.” Nature Reviews Genetics. 2009; 10: 32-42.
- Gräff J, Tsai LH. “Histone acetylation: molecular mnemonics on the chromatin.” Nature reviews Neuroscience. 2013; 14: 97-111.
- Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P. “Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons.” Journal of Cell Science. 2003; 116: 4905-4914.
- Maharana C, Sharma KP and Sharma SK. “Feedback mechanism in depolarization-induced sustained activation of extracellular signal-regulated kinase in the hippocampus.” Scientific reports. 2013; 3: 1103.
- Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. “Regulation of histone acetylation during memory formation in the hippocampus.” Journal of Biological Chemistry. 2004; 279: 40545-40559.
- Latham JA, Dent SY. “Cross-regulation of histone modifications.” Nature structural & molecular biology. 2007; 14: 1017-1024.
- Sui L, Wang Y, Ju LH, Chen M. “Epigenetic regulation of reelin and brainderived neurotrophic factor genes in long-term potentiation in rat medial prefrontal cortex.” Neurobiology of learning and memory. 2012; 97: 425-440.
- McQuown SC, Wood MA. “HDAC3 and the molecular brake pad hypothesis.” Neurobiology of learning and memory. 2011; 96: 27-34.
- De Ruijter AJ, Van GennipAH, Caron HN, Stephan K, Van Kuilenburg AB. “Histone deacetylases (HDACs): characterization of the classical HDAC family.” Biochemical Journal. 2003; 370: 737-749.
- Gregoretti IV, Lee YM, Goodson HV. “Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis.” Journal of molecular biology. 2004; 338: 17-31.
- Broide RS, Redwine JM, Aftahi N, Young W, Bloom FE, Winrow CJ. “Distribution of histone deacetylases 1–11 in the rat brain.” Journal of Molecular Neuroscience. 2007; 31: 47-58.
- Nelson ED, Bal M, Kavalali ET, Monteggia LM. “Selective impact of MeCP2 and associated histone deacetylases on the dynamics of evoked excitatory neurotransmission.” Journal of Neurophysiology. 2011; 106: 193-201.
- Malvaez M, McQuown SC, Rogge GA, Astarabadi M, Jacques V, Carreiro S, et al. “HDAC3-selective inhibitor enhances extinction of cocaine-seeking behavior in a persistent manner.” Proceedings of the national academy of sciences. 2013; 110: 2647-2652.
- Bahari-Javan S, Maddalena A, Kerimoglu C, Wittnam J, Held T, Bähr M, et al. “HDAC1 regulates fear extinction in mice.” Journal of Neuroscience. 2012; 32: 5062-5073.
- Gupta-Agarwal S, Franklin AV, Deramus T, Wheelock M, Davis RL, McMahon LL, et al. “G9a/GLP histone lysine dimethyltransferase complex activity in the hippocampus and the entorhinal cortex is required for gene activation and silencing during memory consolidation.” Journal of Neuroscience. 2012; 32: 5440-5453.
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis- Balboa RC, et al. “Altered histone acetylation is associated with agedependent memory impairment in mice.” Science. 2010; 328: 753-756.
- Takenao Koseki, Akihiro Mouri, Takayoshi Mamiya, Yuki Aoyama,Kazuya Toriumi, Shizuka Suzuki, et al. “Exposure to enriched environments during adolescence prevents abnormal behaviours associated with histone deacetylation in phencyclidine-treated mice.” International Journal of Neuropsychopharmacology. 2012; 15: 1489-1501.
- Sando R, Gounko N, Pieraut S, Liao L, Yates J, Maximov A. “HDAC4 governs a transcriptional program essential for synaptic plasticity and memory.” Cell. 2012; 151: 821-834.
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. “Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear.” Proceedings of the national academy of sciences. 2010; 107: 2675-2680.
- Park H, Poo M. “Neurotrophin regulation of neural circuit development and function.” Nature reviews Neuroscience. 2013; 14: 7-23.
- Psotta L, Lessmann V, Endres T. “Impaired fear extinction learning in adult heterozygous BDNF knock-out mice.” Neurobiology of learning and memory. 2013; 103: 34-38.
- Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. “DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation.” Science. 2003; 302: 890-893.
- Boulle F, van den Hove DL, Jakob SB, Rutten BP, Hamon M, van Os J, et al. Epigenetic regulation of the BDNF gene: implications for psychiatric disorders, Nature Publishing Group. 2012; 17: 584-596.
- Klose RJ, Bird AP. “Genomic DNA methylation: the mark and its mediators.” Trends in biochemical sciences. 2006; 31: 89-97.
- Im HI, Hollander JA, Bali P, Kenny PJ. “MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212.” Nature neuroscience. 2010; 13: 1120-1127.
- Ma DK, Jang MH, Guo JU, Kitabatake Y, Chang ML, Pow-Anpongkul N, et al. “Neuronal activity–induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis.” Science. 2009; 323: 1074-1077.
- Alexi Nott, Marc Watson, Robinson, Luca Crepaldi, Antonella Riccio. “S-Nitrosylation of histone deacetylase 2 induces chromatin remodeling in neurons.” Nature. 2008; 455: 411-415.
- Qureshi IA, Mehler. “Long non-coding RNAs: novel targets for nervous system disease diagnosis and therapy.” Neurotherapeutics. 2013; 10: 632-646.
- Im HI, Kenny PJ. “MicroRNAs in neuronal function and dysfunction.” Trends in neurosciences. 2012; 35: 325-334.
- Fatica A, Bozzoni I. “Long non-coding RNAs: new players in cell differentiation and development.” Nature Reviews Genetics. 2014; 15: 7-21.
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. “A brain-specific microRNA regulates dendritic spine development.” Nature. 2006; 439: 283-289.
- Karr J, Vagin V, Chen K, Ganesan S, Olenkina O, Gvozdev V, et al. “Regulation of glutamate receptor subunit availability by microRNAs.” The Journal of cell biology. 2009; 185: 685-697.
- Saba R, Störchel PH, Aksoy-Aksel A, Kepura F, Lippi G, Plant TD, et al. “Dopamine-regulated microRNA MiR-181a controls GluA2 surface expression in hippocampal neurons.” Molecular and cellular biology. 2012; 32: 619-632.
- Jun Gao, Wen-Yuan Wang, Ying-Wei Mao, Johannes Gräff, Ji-Song Guan, Ling Pan, et al. “A novel pathway regulates memory and plasticity via SIRT1 and miR-134.” Nature. 2010; 466: 1105-1109.
- Rajasethupathy P, Antonov I, Sheridan R, Frey S, Sander C, Tuschl T, et al. “A role for Neuronal piRNAs in the epigenetic control of memory-related synaptic plasticity.” Cell. 2012; 149: 693-707.
- Kurihara M, Shiraishi A, Satake H, Kimura AP. “A conserved noncoding sequence can function as a spermatocyte-specific enhancer and a bidirectional promoter for a ubiquitously expressed gene and a testis-specific long noncoding RNA.” Journal of molecular biology. 2014; 426: 3069-3093.
- Kaur P, Tan JR, Karolina DS, Sepramaniam S, Armugam A, Wong PT, et al. “A long non-coding RNA, BC048612 and a microRNA, miR-203 coordinate the gene expression of neuronal growth regulator 1 (NEGR1) adhesion protein.” Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2016; 1863: 533-543.
- Sennvik K, Benedikz E, Fastbom J, Sundström E, Winblad B, Ankarcrona M. “Calcium ionophore A23187 specifically decreases the secretion of β-secretase cleaved amyloid precursor protein during apoptosis in primary rat cortical cultures.” Journal of neuroscience research. 2001; 63: 429-437.
- Oike Y, Hata A, Mamiya T, Kaname T, Noda Y, Suzuki M, et al. “Truncated CBP protein leads to classical Rubinstein–Taybi syndrome phenotypes in mice: implications for a dominant-negative mechanism.” Human molecular genetics. 1999; 8: 387-396.
- Roelfsema JH, White SJ, Ariyürek Y, Bartholdi D, Niedrist D, Papadia F, et al. “Genetic heterogeneity in Rubinstein-Taybi syndrome: mutations in both the CBP and EP300 genes cause disease.” The American Journal of Human Genetics. 2005; 76: 572-580.
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. “Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl- CpG-binding protein 2.” Nature Genetics. 1999; 23: 185-188.
- Chahrour M, Zoghbi. “The story of Rett syndrome: from clinic to neurobiology.” Neuron. 2007; 56: 422-437.
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, et al. “MeCP2, a key contributor to neurological disease, activates and represses transcription.” Science. 2008; 320: 1224-1229.
- Hagberg B, Aicardi J, Dias K, Ramos O. “A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases.” Annals of neurology. 1983; 14: 471-479.
- Bruck I, Philippart, Giraldi D, Antoniuk S. “Difference in early development of presumed monozygotic twins with Rett syndrome.” American journal of medical genetics. 1991; 39: 415-417.
- Motil KJ, Schultz R, Brown B, Glaze DG, Percy AK. “Altered energy balance may account for growth failure in Rett syndrome.” Journal of child neurology. 1994; 9: 315-319.
- Philip N, Meinecke P, David A, Dean J, Ayme S, Clark R, et al. “Kabuki make-up (Niikawa-Kuroki) syndrome: A study of 62 patients.” American journal of medical genetics. 1988; 31: 565-589.
- Walker FO. “Huntington’s disease.” The Lancet. 2007; 369: 218-228.
- Sananbenesi F, Fischer. “The epigenetic bottleneck of neurodegenerative and psychiatric diseases.” Biological Chemistry. 2009; 390: 1145-1153.
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. “Autophagy fights disease through cellular self-digestion.” Nature. 2008; 451: 1069-1075.
- Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. “Growth factor regulation of autophagy and cell survival in the absence of apoptosis.” Cell. 2005; 120: 237-248.
- Svenning S, Johansen. “Selective autophagy.” Essays in Biochemistry. 2013; 55: 79-92.
- Zaffagnini, G, Martens. “Mechanisms of selective autophagy.” Journal of molecular biology. 2016; 428: 1714-1724.
- Levine B, Kroemer G. “Autophagy in the pathogenesis of disease.” Cell. 2008; 132: 27-42.
- Füllgrabe J, Klionsky DJ, Joseph B. “The return of the nucleus: transcriptional and epigenetic control of autophagy.” Nature reviews Molecular cell biology. 2014; 15: 65-74.
- Shin HJ, Kim H, Oh S, Lee JG, Kee M, Ko HJ, et al. “AMPK–SKP2–CARM1 signaling cascade in transcriptional regulation of autophagy.” Nature. 2016.
- Artal-Martinez de Narvajas A, Gomez TS, Zhang JS, Mann AO, Taoda Y, Gorman JA, et al. “Epigenetic regulation of autophagy by the methyltransferase G9a.” Molecular and cellular biology. 2013; 33: 3983- 3993.
- Wei Y, Xia W, Zhang Z, Liu J, Wang H, Adsay NV, et al. “Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers.” Molecular carcinogenesis. 2008; 47: 701-706.
- Füllgrabe J, Lynch-Day, Heldring Li, Struijk Ma, Hermanson, Rosenfeld, et al. “The histone H4 lysine 16 acetyltransferase hMOF regulates the outcome of autophagy.” Nature. 2013; 500: 468-471.
- White E, Karp C, Strohecker AM, Guo Y, Mathew R. “Role of autophagy in the suppression of inflammation and cancer.” Current opinion in cell biology. 2010; 22: 212-217.
- Wong E, Cuervo AM. “Autophagy gone awry in neurodegenerative diseases.” Nature Neuroscience. 2010; 13: 805-811.
- Menzies FM, Fleming A, Rubinsztein DC. “Compromised autophagy and neurodegenerative diseases.” Nature reviews Neuroscience. 2015; 16: 345- 357.
- Petrovski G, Das DK. “Does autophagy take a front seat in lifespan extension?” Journal of cellular and molecular medicine. 2010; 14: 2543- 2551.
- Gonzalez CD, Lee MS, Marchetti P, Pietropaolo M, Towns R, Vaccaro MI, et al. “The emerging role of autophagy in the pathophysiology of diabetes mellitus.” Autophagy. 2011; 7: 2-11.
- Kim J, Kundu M, Viollet B, Guan KL. “AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1.” Nature cell biology. 2011; 13: 132- 141.
- Cai Z, Yan LJ, Li K, Quazi SH, Zhao B. “Roles of AMP-activated protein kinase in Alzheimer’s disease.” Neuromolecular medicine. 2012; 14: 1-14.
- Duarte AI, Candeias E, Correia SC, Santos RX, Carvalho C, Cardoso S, et al. “Crosstalk between diabetes and brain: glucagon-like peptide-1 mimetics as a promising therapy against neurodegeneration.” Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2013; 1832: 527-541.
- Pugazhenthi S, Qin L, Reddy PH. “Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease.” Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2017; 1863: 1037-1045.
- Flexner JB, Flexner LB, Stellar E. “Memory in mice as affected by intracerebral puromycin.” Science. 1963; 141: 57-59.