
Special Article - Alzheimer’s Disease
Alzheimer’s Disease. Gerontol Geriatr Res. 2017; 3(2): 1032.
Linoleic Acid Derivative DCP-LA Sheds Light on Treatment of Alzheimer’s Disease
Nishizaki T*
Research Section, Innovative Bioinformation Research Organization, Japan
*Corresponding author: Tomoyuki Nishizaki, Research Section, Innovative Bioinformation Research Organization, 2-3-14 Katsuragi, Kita-ku, Kobe, Japan
Received: August 14, 2017; Accepted: September 06, 2017; Published: September 13, 2017
Abstract
Alzheimer’s Disease (AD) is a really tragic disease in which a human being loses human dignity. AD is characterized by extensive deposition of Amyloid β (Aβ) and formation of NFT. To date, none of Aβ-directed drugs for AD therapy have been successful and therefore, a current target for AD therapy focuses on Tau. Glycogen Syntheses Kinase-3β (GSK-3β) is a key protein kinase to phosphorylated Tau. The linoleic acid derivative 8-[2-(2-pentylcyclopropylmethyl)- cyclopropyl]-octanoic acid (DCP-LA) with the cyclopropane ring instead of the cis-double bond selectively activates PKCe, that directly inactivates GSK-3β, leading to suppression of Tau phosphorylation (pTau). DCP-LA-induced PKCe activation, alternatively, directly activates Akt, followed by inactivation of GSK3β, leading to suppression of pTau. DCP-LA also inhibits PTP1B, thereby relatively activating Receptor Tyrosine Kinase (RTK) and Insulin Receptor Substrate 1 (IRS-1), followed by the sequential activation of PhosphoInositide 3-Kinase (PI3K), Phosphoinositide-Dependent Kinase 1 (PDK1), and Akt. Then, activated Akt inactivates GSK-3b, leading to suppression of pTau. DCP-LA bearing synchronous PKCe activation and PTP1B inhibition, thus, could become a promising and novel drug for prevention and treatment of AD.
Keywords: DCP-LA; PKCe; PTP1B; GSK-3β; Alzheimer’s disease; Tau
Introduction
The number of Alzheimer’s Disease (AD) patients is considerably mounting in association with prolongation of life span and AD is currently a major burden on society. No beneficial anti-AD drug, however, has been provided as yet. The most urgent issue, therefore, is to develop drugs for AD.
We have found that cis-unsaturated free fatty acids such as arachidonic, linoleic and linolenic acid, persistently facilitates hippocampal synaptic transmission by targeting presynaptic nicotinic ACh receptor under the control of PKC. Cis-unsaturated free fatty acids, however, are promptly metabolized and decomposed before arriving in the brain, even though the fatty acids are orally or intravenously taken into the body. To resolve this problem, we have synthesized a variety of derivatives of cis-unsaturated free fatty acids, that exhibit stable bioactivities, and obtained the most effective compound 8-[2-(2-pentyl-cyclopropylmethyl)-cyclopropyl]- octanoic acid (DCP-LA), a linoleic acid derivative with cyclopropane rings instead of cis-double bonds [1] (Figure 1).
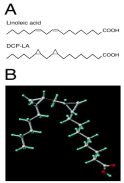
Figure 1: Chemical (A) and conformational structure (B) of DCP-LA.
Beneficial anti-AD drugs require protection of neuronal apoptosis and facilitation of synaptic transmission relevant to cognitive functions. I show here that DCP-LA has the actions of both anti-apoptosis and cognitive enhancement.
Tau Phosphorylation is a Critical Factor for AD
Tau, which is abundantly expressed in neurons of the central nervous system, stabilizes microtubules by interacting with tubulin. When phosphorylated excessively, Tau becomes a trigger for tauopathies [2]. Tauopathies are a class of neurodegenerative diseases associated with aggregation of hyperphosphorylated Tau in an insoluble form in the brain, referred to as Neurofibrillary Tangles (NFT), which include AD, frontotemporal dementia and parkinsonism linked to chromosome 17, progressive supranuclear palsy, Pick’s disease, and corticobasal degeneration. AD is a really tragic disease in which a human being loses human dignity. AD is characterized by extensive deposition of amyloid β (A β) and formation of NFT. To date, none of Aβ-directed drugs for AD therapy have been successful and therefore, a current target for AD therapy focuses on Tau. Of protein kinases proposed glycogen synthese kinase-3β (GSK-3β) is a central executioner to induce Tau phosphorylation (pTau).
Emerging evidence has shown that Aβ serves as an initiator of AD and that Tau acts as an executioner of AD. Mild Cognitive Impairment (MCI) is a preliminary group of AD. Aging, inflammation, and stress activate GSK-3β, to phosphorylate Tau, causing MCI (Figure 2). Then, participation of Aβ further activates GSK-3β and accelerates pTau, leading to progression to AD from MCI (Figure 2). Accordingly, pTau is a key factor both for MCI and AD.
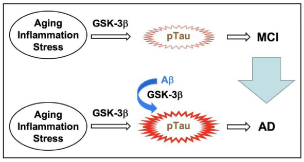
Figure 2: Pathogenesis of MCI and AD.
Interaction of DCP-LA with Protein Kinases and Protein Phosphatases
PKCe
PKC includes the conventional PKC isozymes a, βI, βII, and ?, the novel PKC isozymes d, €, ?, and ?, the atyoical PKC isozymes ?/λ and ?, and the PKC-like isozymes μ and ?. Of PKC isozymes DCP-LA selectively and directly activates PKCe in a Ca2+- and diacylglycerol-independent manner [3]. DCP-LA binds to PKCe at the Phosphatidylserine (PS) binding/associating sites Arg50 and Ile89 in the C2-like domain at the carboxyl-terminal end and the cyclopropane rings, respectively, which is distinct from the binding site (the C1 domain) of phorbol-12-myristate-13-acetate (PMA) [4] (Figure 3). Thus, the primary site of action of DCP-LA is PKCe.
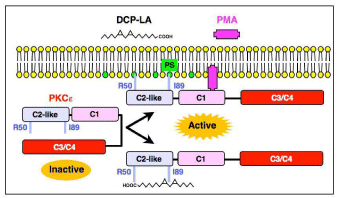
Figure 3: A schematic diagram for DCP-LA-induced PKCe activation.
Protein tyrosine phosphatase 1B (PTP1B) and protein phosphatase 1 (PP1)
DCP-LA inhibits PTP1B [5] and PP1 [6] through its direct interaction.
Akt
Akt1, which is preferentially expressed in neurons, is activated by being phosphorylated at Thr308 and Ser473. DCP-LA-induced PTP1B inhibition relatively activates Receptor Tyrosine Kinase (RTK) and Insulin Receptor Substrate 1 (IRS-1), followed by activation of Akt through a pathway along a RTK/IRS-1/phosphoinositide 3-kinase (PI3K)/phosphoinositide- dependent kinase 1 (PDK1)/Akt axis [7] (Figure 4). PKCe, activated by DCP-LA, directly activates Akt1 by phosphorylating at Ser473 [7] (Figure 4).
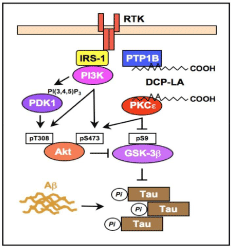
Figure 4: A schematic pathway underlying DCP-LA-induced Akt activation
and GSK-3b inactivation.
GSK-3β
GSK-3β is activated and inactivated by being phosphorylated at Tyr216 and Ser9, respectively. Akt, that is activated by PKCe or through a RTK/IRS-1/PI3K/PDK1/Akt pathway in association with PTP1B inhibition under the influence of DCP-LA, inactivates GSK- 3β by phosphorylating at Ser9 [7] (Figure 4).
PKCe, activated by DCP-LA, inactivates GSK-3β by directly phosphorylating at Ser9 [7] (Figure 4).
DCP-LA Suppresses Tau Phosphorylation
Taken together, DCP-LA is capable of suppressing pTau by inactivating GSK-3β [7] (Figure 4). This indicates that DCP-LA has the potential to prevent and improve tauopathies including MCI and AD.
DCP-LA Protects Neuronal Cells from Apoptosis
Neuronal apoptosis in the neurodegenerative diseases such as AD and Parkinson’s disease is caused by Endoplasmic Reticulum (ER) stress or oxidative stress. DCP-LA inhibits Nitric Oxide (NO) stressinduced activation of caspase-3/-9 and apoptosis of neuronal cells [8]. This indicates that DCP-LA could prevent or delay progression of AD.
DCP-LA Facilitates Synaptic Transmission
Long-term Potentiation (LTP) is an established cellular model of learning and memory. DCP-LA activates presynaptic PKCe (Figure 5A-?), which promotes vesicular transport of a7 ACh receptor (a7R) towards the cell surface from the cytosol (Figure 5A-?), to increase a7R on the plasma membrane at the presynaptic terminals (Figure 5A-?). An increase in the number of a7R allows greater deal of Ca2+ influx (Figure 5A-?), which stimulates glutamate release from presynaptic terminals (Figure 5A-?), thereby facilitating synaptic transmission, leading to LTP [1,9-11].
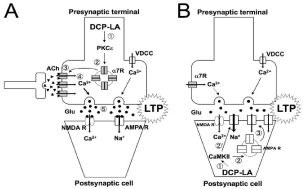
Figure 5: DCP-LA-induced LTP pathway.
NMDA R: N-Methyl-D-Aspartate Receptor; VDCC: Voltage-Dependent
Calcium Channel.
DCP-LA, alternatively, indirectly activates Ca2+/calmodulindependent protein kinase II (CaMKII) due to PP1 inhibition (Figure 5B-?), which triggers vesicular transport of a-Amino-3-hydroxy- 5-Methyl-4-isoxazolepropionic Acid (AMPA) Receptor (AMPA R) towards the plasma membrane from the cytosol (Figure 5B-?), to increase cell surface localization of AMPA R at the postsynaptic cells (Figure 5B-?). In addition, CaMKII potentiates AMPA R responses by phosphorylating AMPA R (Figure 5B-?). An increase in the number of AMPA R at the postsynaptic cells and potentiation of AMPA R responses enhance total excitatory postsynaptic conductance, leading to LTP [6]. DCP-LA, thus, could enhance cognitive functions by inducing LTP.
In addition, DCP-LA stimulates presynaptic release of ?-aminobutyric acid (GABA), serotonin, and dopamine by targeting a7R [12-14]. This implies that DCP-LA could tune not only cognitive functions but other brain functions.
DCP-LA Ameliorates Cognitive Impairment
Lines of evidence have shown that DCP-LA improves Aβ1- 40 - or mutant Aβ-induced spatial learning deficits in rats [15,16], scopolamine-induced spatial learning and memory disorders in rats [15], spatial learning and memory deterioration in senescence accelerated mice (SAMP8) [17,18], and spatial learning and memory impairment in 5xFAD transgenic mice, an animal model of AD [7].
Conclusion
DCP-LA is capable of efficiently suppressing Tau phosphorylation, responsible for MCI and AD. DCP-LA has the potential to inhibit oxidative stress-induced caspase activation and neuronal apoptosis, responsible for brain atrophy in AD. In addition, DCP-LA could ameliorate cognitive impairment in association with AD by facilitating synaptic transmissions. Consequently, DCP-LA must become a promising therapeutic drug of AD and relieve huge number of AD patients from despair.
References
- Tanaka A, Nishizaki T. The newly synthesized linoleic acid derivative FR236924 induces a long-lasting facilitation of hippocampal neurotransmission by targeting nicotinic acetylcholine receptors. Bioorg Med Chem Lett. 2003; 13: 1037-1040.
- Josephs KA. Current understanding of neurodegenerative diseases associated with the protein tau. Mayo Clin Proc. 2017; 92: 1291-1303.
- Kanno T, Yamamoto H, Yaguchi T, Hi R, Mukasa T, Fujikawa H, et al. The linoleic acid derivative DCP-LA selectively activates PKC-e, possibly binding to the phosphatidylserine binding site. J Lipid Res. 2006; 47: 1146-1156.
- Kanno T, Tsuchiya A, Shimizu T, Mabuchi M, Tanaka A, Nishizaki T. DCPLA activates cytosolic PKCe by interacting with the phosphatidylserine binding/associating sites Arg50 and Ile89 in the C2-like domain. Cell Physiol Biochem. 2015; 37: 193-200.
- Tsuchiya A, Kanno T, Nagaya H, Shimizu T, Tanaka A, Nishizaki T. PTP1B inhibition causes Rac1 activation by enhancing receptor tyrosine kinase signaling. Cell Physiol Biochem. 2014; 33: 1097-1106.
- Kanno T, Yaguchi T, Nagata T, Tanaka A, Nishizaki T. DCP-LA stimulates AMPA receptor exocytosis through CaMKII activation due to PP-1 inhibition. J Cell Physiol. 2009; 221: 183-188.
- Kanno T, Tsuchiya A, Tanaka A, Nishizaki T. Combination of PKCe activation and PTP1B inhibition effectively suppresses Ab-induced GSK-3b activation and Tau phosphorylation. Mol Neurobiol. 2016; 53: 4787-4797.
- Yaguchi T, Fujikawa H, Nishizaki T. Linoleic acid derivative DCP-LA protects neurons from oxidative stress-induced apoptosis by inhibiting caspase-3/-9 activation. Neurochem Res. 2010; 35: 712-717.
- Yamamoto S, Kanno T, Nagata T, Yaguchi T, Tanaka A, Nishizaki T. The linoleic acid derivative FR236924 facilitates hippocampal synaptic transmission by enhancing activity of presynaptic a7 acetylcholine receptors on the glutamatergic terminals. Neuroscience. 2005; 130: 207-213.
- Kanno T, Tanaka A, Nishizaki T. Linoleic acid derivative DCP-LA stimulates vesicular transports of a7 ACh receptors towards surface membrane. Cell Physiol Biochem. 2012; 30: 75-82.
- Kanno T, Tsuchiya A, Tanaka A, Nishizaki T. The linoleic acid derivative DCP-LA increases membrane surface localization of the a7 ACh receptor in a protein 4.1N-dependent manner. Biochem J. 2013; 450: 303-309.
- Kanno T, Yaguchi T, Yamamoto S, Yamamoto H, Fujikawa H, Nagata T, et al. 8-[2-(2-Pentyl-cyclopropylmethyl)-cyclopropyl]-octanoic acid stimulates GABA release from interneurons projecting to CA1 pyramidal neurons in the rat hippocampus via pre-synaptic a7 acetylcholine receptors. J Neurochem. 2005; 95: 695-702.
- Shimizu T, Kanno T, Tanaka A, Nishizaki T. a,b-DCP-LA selectively activates PKC-e and stimulates neurotransmitter release with the highest potency among 4 diastereomers. Cell Physiol Biochem. 2011; 27: 149-158.
- Kanno T, Tanaka A, Nishizaki T. Linoleic acid derivative DCP-LA ameliorates stress-induced depression-related behavior by promoting cell surface 5-HT1A receptor translocation, stimulating serotonin release, and inactivating GSK-3b Mol Neurobiol. 2015; 51: 523-532.
- Nagata T, Yamamoto S, Yaguchi T, Iso H, Tanaka A, Nishizaki T. The newly synthesized linoleic acid derivative DCP-LA ameliorates memory deficits in animal models treated with amyloid-b peptide and scopolamine. Psychogeriatrics. 2005; 5: 122-126.
- Nagata T, Tomiyama T, Mori H, Yaguchi T, Nishizaki T. DCP-LA neutralizes mutant amyloid b peptide-induced impairment of long-term potentiation and spatial learning. Behav Brain Res. 2010; 206: 151-154.
- Yaguchi T, Nagata T, Mukasa T, Fujikawa H, Yamamoto H, Yamamoto S, et al. Linoleic acid derivative DCP-LA improves learning impairment in SAMP8. Neuroreport. 2006; 17: 105-108.
- KannoT, YaguchiT, ShimizuT, TanakaA, NishizakiT. 8-[2-(2-Pentylcyclopropylmethyl)- cyclopropyl]-octanoic acid and its diastereomers improve age-related cognitive deterioration. Lipids. 2012; 47: 687-695.