
Special Article - Geriatric Medicine
Gerontol Geriatr Res. 2017; 3(2): 1033.
Physician Referral Patterns Impact Survival of Older Women with Metastatic Breast Cancer
Tkaczuk KHR¹*, Yong C¹, Onukwugha E¹, Mullins CD¹ and Hussain A¹,²
¹University of Maryland School of Medicine, USA
²Veterans Affairs Medical Center, USA
*Corresponding author: Katherine HR Tkaczuk, University of Maryland School of Medicine, Professor of Medicine, Director, Breast Evaluation and Treatment Program, University of Maryland Greenebaum Comprehensive Cancer Center, 22 South Greene St, Baltimore, USA
Received: August 09, 2017; Accepted: November 22, 2017; Published: November 29, 2017
Abstract
Objective: Contact with Primary Care Physicians (PCP) has been found to be associated with improved outcomes, including decreased mortality, among women with breast cancer. The impact of the medical oncologist in the context of PCP contact on outcomes of women with metastatic BC (mBC) is not well defined. We examined if PCP contact prior to mBC diagnosis affects survival independently of or is in some measure dependent on subsequent medical oncologist contact.
Methods: This analysis used linked SEER (Surveillance, Epidemiology, and End Results) and Medicare data on women aged 65+ with incident mBC diagnosed during 2007-2009. Using Cox proportional hazards models, we examined the influence of PCP contact within the one-year period preceding mBC diagnosis on the probability of post-diagnosis medical oncologist visit and on overall mortality, adjusting for baseline patient characteristics.
Results: Of 2,066 women (mean age 77 years), 728 (35.2%) did not have PCP contact within one year before mBC diagnosis. Women with three or more PCP visits were more likely to see a medical oncologist (3-4 visits vs. 0 visits: HR=1.29; 95% CI=1.11-1.50; 5+ visits vs 0 visits: HR=u1.41; 95% CI=1.22- 1.63). Older age, African American race, and lower socioeconomic status were statistically significantly associated with decreased probability of medical oncologist visit. All-cause mortality was lower among those with post-diagnosis medical oncologist contact (53.5% - 55.8%), and higher (86.7% - 89.8%) among those without medical oncologist contact (p<0.01).
Conclusion: Pre-diagnosis primary care contact impacts positively referral to medical oncology subspecialty care while the post-diagnosis access to medical oncology care contributes significantly to lower all-cause mortality in older patients with incident mBC.
Keywords: Metastatic breast cancer; Primary care; Physician specialist; Medical oncology
Abbreviations
AJCC-TNM: American Joint Committee on Cancer Tumor- Node-Metastasis staging; AMA: American Medical Association; CCI: Charlson Comorbidity Index; CI: Confidence Interval; ER: Estrogen Receptor; HR: Hazard Ratio; IQR: Interquartile Range; mBC: Metastatic Breast Cancer; PCP: Primary Care Physician; PR: Progesterone Receptor; SD: Standard Deviation; SEER: Surveillance, Epidemiology, and End Results Cancer Registry
Introduction
Compared to early stage breast cancer, metastatic Breast Cancer (mBC) is associated with poorer prognosis with a median overall survival of 2 to 3 years [1-3]. Although mBC is not considered curable, it is generally treatable with the primary goals of care being to optimize survival time and quality of life [4]. Comprehensive care for women with breast cancer generally includes evaluation and management by a multidisciplinary team, including medical oncologists, radiation oncologists, breast surgeons as well as Primary Care Physicians (PCP) [5,6]. Barriers or delays to cancer specialist referral following diagnosis of cancer could have negative implications on patient satisfaction with care [7], treatment receipt [8,9], and clinical outcomes such as overall survival [10].
Although patient referral to a medical oncologist for cancer treatment evaluation is considered a key step in the pathway to treatment receipt [8,9,11], there is limited published evidence on the factors that influence referral to medical oncologists, particularly in the advanced breast cancer setting. The potential barriers to patient referral to specialty oncology care include patient-level factors such as socioeconomic constraints, and system-level factors such as restricted provider networks and preauthorization requirements [12]. In addition to these factors, an individual’s ability to engage in the healthcare system during the pre-diagnosis period, including regular contact with a PCP, could also influence referral to specialty care following their cancer diagnosis.
Previous studies have reported that PCPs play an important role in the early detection of breast cancer through cancer screening [13,14]. Increasing number of PCP visits in the period prior to BC diagnosis has been found to be associated with improved BC-related outcomes, including lower odds of late-stage diagnosis, and lower BC-specific and overall mortality [15,16]. Specifically, among 90,537 Medicare women with BC diagnosed during the years 1994-2005, women who had 10 or more PCP visits in the 24-month period prior to BC diagnosis had 41% lower BC mortality, and 27% lower overall mortality, compared with women who had 0 to 1 PCP visit [15]. However, it is uncertain if primary care in the pre-diagnosis period influences mortality independently, or through the effect of primary care on referral to medical oncology. Therefore, we undertook the present study to address the question of whether PCP contact with in one year prior to incident (newly diagnosed) mBC affects BC outcomes independently or is in some measure dependent on subsequent medical oncologist contact. The potential role of the PCP in this context would be particularly relevant to the elderly women population since PCPs are likely to play an increasingly important role in the healthcare of aging women, and the management of patients with cancer [17].
The study objective was to examine the association between prediagnosis PCP contact and post-diagnosis medical oncologist visit among older women with newly diagnosed mBC, and the influence of physician contact on overall survival. We hypothesized that women with primary care contact in the pre-diagnosis period were more likely to visit a medical oncologist after mBC diagnosis. We also explored whether the intensity of primary care contact (number of pre-diagnosis PCP visits) had any influence on the probability of post-diagnosis medical oncologist visit.
Patients and Methods
Study design and study population
This was a retrospective cohort analysis of linked Surveillance, Epidemiology, and End Results cancer registry and Medicare claims data (SEER-Medicare) on female Medicare beneficiaries with incident mBC diagnosed during January 2007 to December 2009. The study was approved by the University of Maryland Institutional Review Board. Stage of breast cancer was determined from SEER in accordance with the American Joint Committee on Cancer Tumor-Node-Metastasis (AJCC-TNM) staging, 6th edition [18]. Patients were included in the study sample if they were age 66 or older at the time of diagnosis, had continuous enrollment in Medicare Parts A and B in the 12 months prior to diagnosis month, and survived for at least 30 days after diagnosis. Patients were excluded if they had any of the following: 1) history of any cancer (excluding non-melanoma skin cancer) within 5 years prior to the BC diagnosis; 2) unknown diagnosis month or year; or 3) incident post-mortem BC diagnosis. Medicare claims data from 2006 to 2011 were used to capture information on physician visits during the pre-diagnosis and post-diagnosis periods.
Variables
The primary outcomes were post-diagnosis medical oncologist visit, and overall mortality following diagnosis. The independent variable of interest was a binary indicator for any PCP visits during the one year pre-diagnosis period (0 PCP visits vs. 1 or more visits). To explore if the intensity of primary care contact affected referral to a medical oncologist, PCP visits in the pre-diagnosis period was categorized as follows: 0 PCP visits vs. 1-2 visits vs. 3-4 visits vs. 5 or more visits.
Medicare claims from the Carrier Claims (National Claims History) and Outpatient files were used to capture physician visits in the ambulatory setting. Physician specialty was determined from the American Medical Association (AMA) Physician Masterfile. Medical oncologists were identified from AMA specialty codes for hematology and/or oncology. PCPs were identified from specialty codes for general practice, family practice, internal medicine, or geriatric medicine. Radiation oncologists and surgical oncologists were identified based on specialty codes for radiation oncology and surgical oncology, respectively.
Statistical analyses
Descriptive characteristics of the study sample were presented using frequency distributions for categorical variables and median and inter quartile values for continuous variables. Chi-square tests were used to determine the bivariate associations between patient characteristics and any pre-diagnosis PCP visits. Cox proportional hazards models were estimated to examine the covariate-adjusted association between pre-diagnosis PCP contact and the probability of post-diagnosis medical oncologist visit. We also estimated separate Cox proportional hazards models for all-cause mortality in the full sample and among patients with at least 1 medical oncologist visit to determine if pre-diagnosis PCP contact had any additional effect on overall survival, conditional on a medical oncologist visit.
The following potential confounding demographic, clinical, and contextual variables were included in the regression models: age group at diagnosis, race/ethnicity, Estrogen Receptor/Progesterone Receptor (ER/PR) status at diagnosis, tumor differentiation (poorly or undifferentiated tumor), comorbidity burden at baseline as measured by the Charlson Comorbidity Index (CCI), screening and preventive care services (including screening/diagnostic mammography, and flu vaccination) in the year prior to diagnosis, a single proxy measure for poor performance status (any use of wheelchair, walking aid, oxygen, skilled nursing facility service, or hospitalization in the year prior to diagnosis), diagnosis year, and census region of SEER registry (Northeast, West, Midwest, or South). The regression models also included a proxy measure for low income i.e., an indicator for any state buy-in in the year prior to diagnosis. Medicare buy-in benefits are generally operated by state Medicaid programs, and are provided to low-income Medicare beneficiaries to cover their Medicare premiums, deductibles, and copayments [19].
Interaction terms between pre-PCP visits and patient factors including age, race, and state buy-in were included in the Cox proportional hazard models to test for the presence of statistically significant interactions. The final regression model did not include any interaction terms as there were no statistically significant interactions identified. The proportional hazard assumption with respect to the pre-PCP visits variable was also tested. Time-invariant adjusted Hazard Ratios (HR) was reported as the proportional hazard assumption was not violated. All statistical tests were two-tailed with a 0.05 cut-off value for statistical significance. All statistical analysis was conducted using Version 9.3 of the SAS System.
Results
Descriptive characteristics of study cohort
The study cohort included 2,066 female Medicare recipients with incident metastatic breast cancer diagnosed during 2007 to 2009. The mean age of the study sample was 77 years, and the sample comprised 81% who were non-Hispanic white and 11% who were non-Hispanic African American. Of the 2,066 women, 728 (35.2%) did not have any PCP visit during the one year prior to diagnosis. Among the 1,338 women with at least 1 PCP visit in the year prior to diagnosis, the average number of PCP visits was 5 visits (SD=3.8; Inter Quartile Range (IQR) = 2-6 visits). Descriptive characteristics of the sample, stratified by any PCP visits in the one year prior to diagnosis, are shown in Table 1. Overall, compared to those with at least 1 PCP visit, patients with no PCP visits in the pre-diagnosis period were younger, had lower comorbidity burden as measured by the Charlson Comorbidity Index (CCI), and had a lower proportion with poor performance status (any use of wheelchair, walking aid, oxygen, skilled nursing facility service, or hospitalization in the year prior to diagnosis) (p<0.01 for all).
Overall
No PCP visit pre-diagnosis
At least 1 PCP visit
(N=2066)
(N=728)
(N=1338)
N
(%)
N
(%)
N
(%)
p-value
Age
<0.01
66-74
843
(41.8)
330
(45.3)
513
(38.3)
75-84
814
(39.4)
265
(36.4)
549
(41.)
85+
409
(19.8)
133
(18.3)
276
(20.6)
Race
0.11
Non-Hispanic White
1674
(81.0)
575
(79.0)
1099
(82.1)
Non-Hispanic African American
235
(11.4)
97
(13.3)
138
(10.3)
Other
157
(7.6)
56
(7.7)
101
(7.5)
ER/PR status
0.79
ER +ve (PR +ve or –ve)
1268
(61.4)
441
(60.6)
827
(61.8)
ER –ve (PR +ve or –ve)
398
(19.3)
146
(20.1)
252
(18.8)
ER/PR unknown
400
(19.4)
141
(19.4)
259
(19.4)
Charlson Comorbidity Index
<0.0001
Zero
1337
(64.7)
559
(76.8)
778
(58.1)
One
402
(19.5)
78
(10.7)
324
(24.2)
Two or higher
327
(15.8)
91
(12.5)
236
(17.6)
Poor performance status proxy*
465
(22.5)
119
(16.4)
346
(25.9)
<0.0001
Screening and preventive care pre-diagnosis
Screening mammography
274
(13.3)
38
(5.2)
236
(17.6)
<0.0001
Diagnostic mammography
158
(7.6)
28
(3.8)
130
(9.7)
<0.0001
Flu vaccination
811
(39.3)
151
(20.7)
660
(49.3)
<0.0001
Low income proxy**
362
(17.5)
143
(19.6)
219
(16.4)
0.06
Specialist visits (1 year post-diagnosis)
Medical oncologist
1595
(77.2)
512
(70.3)
1083
(80.9)
<0.0001
Primary care physician
1411
(68.3)
298
(40.9)
1113
(83.2)
<0.0001
Radiation oncologist
614
(29.7)
204
(28.0)
410
(30.6)
0.21
Surgical oncologist
99
(4.8)
32
(4.4)
67
(5.0)
0.53
Table 1: Descriptive characteristics of women diagnosed with metastatic breast cancer in 2007-2009, stratified by Primary Care Physician (PCP) visits in the 1 year pre-diagnosis (N=2,066).
The proxy measure of Poor Performance Status (PPSP) varied with patient age at diagnosis. The overall proportion of patients with PPSP at baseline was 22.5% and the proportion was highest among those aged 85 and older (29.8%; 25.3% among those aged 75 to 84 years; 16.3% among those aged 66 to 74 years; p<0.01). The components of the PPSP measure differed between age groups for all components except the indicator for oxygen tank use prior to diagnosis (data not shown). While 16% of patients without a PCP visit pre-diagnosis had a PPSP, we cannot determine from the available data whether the presence of PPSP prevented a visit to the PCP. However we do note that the positive association between the PPSP measure and a PCP visit is driven by the indicator for a hospitalization prior to diagnosis and, to a lesser extent, by the indicator for a walking aid claim during the pre-diagnosis period (data not shown).
Further, those with no PCP visits in the pre-diagnosis period had lower rates of screening/diagnostic services and preventive care including screening mammography, diagnostic mammography, and flu vaccination (p<0.01 for all).
Bivariate associations between primary care contact and specialist visits
Table 1 also shows the proportion of patients with specialist visits in the post-diagnosis period. The proportion with any medical oncologist visit in the 1 year post-diagnosis period was 70.3% among those with no pre-diagnosis PCP visits, and 10 percentage points higher (80.9%) among those with at least 1 pre-diagnosis PCP visit (p<0.01). Overall, 1,083 (52%) of the sample had a pre-diagnosis PCP visit followed by a post-diagnosis medical oncologist visit, 255 (12%) had a pre-diagnosis PCP visit only, 512 (25%) had no pre-diagnosis PCP visit but had a post-diagnosis medical oncologist visit, and 216 (10%) had no evidence of pre-diagnosis PCP visit or post-diagnosis medical oncologist visit.
The proportion of patients with post-diagnosis PCP visits was also higher among those with at least 1 pre-diagnosis PCP visit (83.2% vs. 40.9% for those with no pre-diagnosis PCP visits, p<0.01). There was no association between pre-diagnosis PCP visits and post-diagnosis visits with radiation oncologists (p=0.21) or surgical oncologists (p=0.53). As shown in Figure 1, the all-cause mortality was lowest among those with post-diagnosis medical oncologist contact (53.5% - 55.8%), and highest (86.7% - 89.8%) among those without medical oncologist contact (p<0.01).
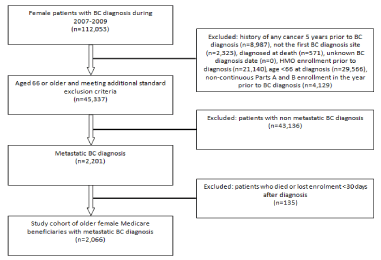
Figure 1: Cohort selection of older female medicare beneficiaries with metastatic breast cancer (N=2,066).
Multivariable regression results
The adjusted Hazards Ratios (HR) for time to post-diagnosis medical oncologist visit are shown in Table 2, with HR greater than 1 indicating greater probability of medical oncologist visit compared to the referent category. Compared to women with 0 pre-diagnosis PCP visits, those with 1 or more pre-diagnosis visits were more likely to see a medical oncologist (HR=1.23; 95% CI=1.09-1.37). (Table 2, left column)
Model examining
Model examining
0 vs. 1+ PCP visits
number of PCP visits
Variable
HR
(95% CI)
p-value
HR
(95% CI)
p-value
Number of PCP visits pre-diagnosis
0 visits
Reference
-
-
-
1 or more visits
1.2
(1.09 - 1.37)
<0.01
-
-
-
Number of PCP visits pre-diagnosis
0 visits
-
-
-
Reference
1-2 visits
-
-
-
1.1
(0.94 – 1.24)
0.31
3-4 visits
-
-
-
1.3
(1.11 – 1.50)
<0.01
5 or more visits
-
-
-
1.4
(1.22 – 1.63)
<0.0001
Age
66-74
Reference
Reference
75-84
0.8
(0.75 - 0.93)
<0.01
0.8
(0.75 - 0.93)
<0.01
85+
0.6
(0.52 - 0.70)
<0.0001
0.6
(0.52 - 0.70)
<0.0001
Race
Non-Hispanic White
Reference
Reference
Non-Hispanic African American
0.8
(0.68 - 0.96)
0.01
0.8
(0.69 - 0.97)
0.02
Other
1
(0.84 - 1.25)
0.82
1
(0.83 - 1.24)
0.89
ER/PR status
ER +ve (PR +ve or –ve)
Reference
Reference
ER -ve(PR +ve or –ve)
0.9
(0.82 - 1.06)
0.3
0.9
(0.83 - 1.08)
0.37
ER/PR unknown
0.6
(0.53 - 0.73)
<0.0001
0.6
(0.53 - 0.73)
<0.0001
Charlson Comorbidity Index
Zero
Reference
Reference
One
1.1
(0.94 - 1.23)
0.3
1
(0.91 - 1.20)
0.32
Two or higher
0.9
(0.75 - 1.05)
0.15
0.8
(0.71 - 1.00)
0.05
Proxy for low income
Not low income
Reference
Reference
Low income*
0.8
(0.70 - 0.93)
<0.01
0.8
(0.69 - 0.92)
<0.01
Regression model also controlled for poor tumor differentiation, screening/preventive care during the year prior to diagnosis (screening mammography, diagnostic mammography, flu vaccination), poor performance status proxy during the year prior to diagnosis (indicator for any use of SNF, hospitalization, walking aids, wheelchairs, or home oxygen), year of diagnosis, and census region of SEER registry.
*Low income individuals had at least 1 month participation in a state buy-in program in the 1 year pre-diagnosis. State buy-in programs pay for Medicare expenses, such as Parts A and/or B premiums, for low-income individuals.
Table 2: Adjusted Hazards ratios (HR) for post-diagnosis medical oncologist visit among women diagnosed with incident metastatic breast cancer in 2007-2009 (N=2,066) [HR greater than 1 indicates greater probability of medical oncologist visit following diagnosis compared to reference category].
Importantly, compared to the youngest age group of patients (66- 74 years old at diagnosis), the older age groups i.e. 75-84 years and 85+ years had lower probability of medical oncologist visit; the HRs (95% CI) were 0.84 (0.75 - 0.93) and 0.61 (0.52 - 0.70), respectively. Women who were non-Hispanic African American also had lower hazard of medical oncologist visit compared to women who were non-Hispanic white (HR=0.81; 95% CI=0.68 - 0.96). Patients with ER/PR status unknown had lower probability of medical oncologist visit compared to those with ER positive tumors (HR=0.62; 95% CI=0.53-0.73). The results also suggested that patient’s socioeconomic status influenced referral to medical oncologist. Women with at least 1 month of state buy-in in the year prior to diagnosis (i.e., low income) had statistically significant lower probability of medical oncologist visit compared to those with no state buy-in (HR=0.80; 95% CI=0.70 - 0.93).
To explore if the intensity of primary care contact had any influence on the time to post-diagnosis medical oncologist visit, we ran regression models with pre-diagnosis PCP visits categorized as follows: 0 PCP visits vs. 1-2 visits vs. 3-4 visits vs. 5 or more visits (Table 2, right column). The results indicate that the probability of medical oncologist visit was not statistically significantly different among those with 0 PCP visits or 1-2 PCP visits. On the other hand, compared to those with 0 pre-diagnosis PCP visits, the probability of post-diagnosis medical oncologist visit was higher among those with 3-4 PCP visits and 5 or more PCP visits; the HRs (95% CI) were 1.29 (1.11 - 1.50) and 1.41 (1.22 - 1.63), respectively.
As shown in Table 3, in the full sample (n=2,066), women with 1 or more pre-diagnosis PCP visits had lower all-cause mortality compared to women with 0 pre-diagnosis PCP visits (HR=0.85; 95% CI=0.76-0.96). However, when the all-cause risk of mortality analysis was limited to the subgroup of 1,595 women with at least 1 post-diagnosis medical oncologist visit, the adjusted HR for all-cause mortality was not statistically significantly different among those with 0 pre-diagnosis PCP visits vs. 1 or more pre-diagnosis PCP visits (HR=1.00; 95% CI=0.86 - 1.17) (Table 4), which suggests that PCP contact positively influences referral to medical oncologist, and it is the subsequent access to medical oncology care that primarily leads to lower mortality in women with mBC (Figure 2).
Model examining pre-diagnosis PCP visits and post-diagnosis medical oncologist visit
Model examining pre-diagnosis PCP visits only
Variable
HR
(95% CI)
p-value
HR
(95% CI)
p-value
Number of PCP visits pre-diagnosis
0 visits
Reference
Reference
1 or more visits
0.9
(0.82 - 1.05)
0.22
0.9
(0.76 - 0.96)
0.01
Medonc visit in 1 year post-diagnosis
0 visits
Reference
-
-
-
1 or more visits
0.3
(0.29 - 0.38)
<.0001
-
-
-
Age
66-74
Reference
Reference
75-84
1.3
(1.14 - 1.48)
<0.01
1.4
(1.26 - 1.62)
<.0001
85+
1.4
(1.16 - 1.60)
<0.01
1.8
(1.52 - 2.07)
<.0001
Race
Non-Hispanic White
Reference
Reference
Non-Hispanic African American
0.9
(0.79 - 1.13)
0.52
1
(0.82 - 1.17)
0.81
Other
0.8
(0.63–1.00)
0.05
0.8
(0.65 - 1.03)
0.09
ER/PR status
ER +ve (PR +ve or –ve)
Reference
Reference
ER -ve(PR +ve or –ve)
2
(1.75–2.35)
<.0001
2
(1.76–2.36)
<.0001
ER/PR unknown
1.6
(1.33 - 1.81)
<.0001
1.9
(1.64 - 2.20)
<.0001
Tumor differentiation
Not poorly differentiated tumor
Reference
Reference
Poorly or undifferentiated tumor
1.3
(1.14 - 1.54)
<0.01
1.3
(1.12 - 1.51)
<0.01
Unknown
1.4
(1.16 - 1.58)
<.0001
1.4
(1.22 - 1.64)
<.0001
Charlson Comorbidity Index
Zero
Reference
Reference
One
1.1
(0.90 - 1.22)
0.56
1
(0.88 - 1.19)
0.12
Two or higher
1.2
(1.01 - 1.41)
0.04
1.2
(1.03 - 1.44)
0.02
Screening and preventive care pre-diagnosis
No screening mammography
Reference
Reference
Screening mammography
0.8
(0.65 - 0.94)
0.01
0.7
(0.60 - 0.87)
<0.01
Other pre-diagnosis measures
No poor performance status proxy
Reference
Reference
Poor performance status proxy*
1.2
(1.08 - 1.43)
<0.01
1.3
(1.13 - 1.51)
<0.01
Not low income
Reference
Reference
Low income**
1.3
(1.14 - 1.53)
<0.01
1.4
(1.17 - 1.58)
<.0001
Regression model also controlled for diagnostic mammography, pre-diagnosis flu vaccination, year of diagnosis and census region of SEER registry.
*Poor performance status proxy in the 1 year prior to diagnosis was measured by an indicator for any use of SNF, hospitalization, walking aids, wheelchairs, or home oxygen.
**Low income individuals had at least 1 month participation in a state buy-in program in the 1 year pre-diagnosis. State buy-in programs pay for Medicare expenses, such as Parts A and/or B premiums, for low-income individuals.
Table 3: Adjusted Hazards Ratios (HR) for all-cause mortality among women with metastatic breast cancer (N=2,066) [HR greater than 1 indicates increased mortality risk compared to reference category].
All-cause mortality
Variable
HR
(95% CI)
p-value
Number of PCP visits pre-diagnosis
0 visits
Reference
1 or more visits
1
(0.86 - 1.17)
0.99
Age
66-74
Reference
75-84
1.4
(1.23 - 1.66)
<.0001
85+
1.8
(1.45 - 2.14)
<.0001
Race
Non-Hispanic White
Reference
Non-Hispanic African American
0.9
(0.73 - 1.14)
0.44
Other
0.9
(0.67 - 1.16)
0.36
ER/PR status
ER +ve (PR +ve or –ve)
Reference
ER -ve(PR +ve or –ve)
2.1
(1.77 - 2.47)
<.0001
ER/PR unknown
1.6
(1.33–1.97)
<.0001
Tumor differentiation
Not poorly differentiated tumor
Reference
Poorly or undifferentiated tumor
1.4
(1.17 - 1.64)
<0.01
Unknown
1.3
(1.11 - 1.59)
<0.01
Charlson Comorbidity Index
Zero
Reference
One
1.1
(0.93 - 1.32)
0.22
Two or higher
1.2
(0.96 - 1.45)
0.13
Screening and preventive care pre-diagnosis
No screening mammography
Reference
Screening mammography
0.8
(0.64 - 0.96)
0.02
Other pre-diagnosis measures
No poor performance status proxy*
Reference
Poor performance status proxy
1.3
(1.10 - 1.57)
<0.01
Not low income
Reference
Low income**
1.4
(1.14 - 1.64)
<0.01
Regression model also controlled for diagnostic mammography, pre-diagnosis flu vaccination, year of diagnosis and census region of SEER registry.
*Poor performance status proxy in the 1 year prior to diagnosis was measured by an indicator for any use of SNF, hospitalization, walking aids, wheelchairs, or home oxygen.
**Low income individuals had at least 1 month participation in a state buy-in program in the 1 year pre-diagnosis. State buy-in programs pay for Medicare expenses, such as Parts A and/or B premiums, for low-income individuals.
Table 4: Adjusted hazard ratios (HR) for all-cause mortality among women with at least 1 medical oncologist visit (N=1,595) [HR greater than 1 indicates increased mortality risk compared to reference category].
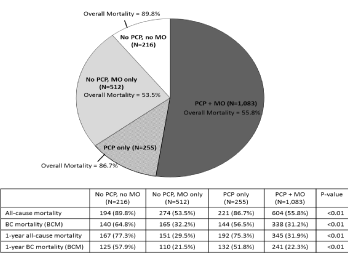
Figure 2: Overall all-cause mortality among women with metastatic breast cancer, by whether they had any pre-diagnosis primary care physician (PCP) visit and/
or post-diagnosis medical oncologist (MO) visit (N=2,066).
Discussion
In the current study we report for the first time on the connection between frequencies of the primary care follow up in elderly female Medicare recipients before the diagnosis of incident mBC and subsequent referral patterns to medical oncology subspecialty care. Patients without pre-diagnosis PCP contact were younger than those with at least 1 PCP visit, given their older age; we would expect patients with PCP contact to have poorer survival, independent of other factors. However, in adjusted analysis, we found that patients with PCP contact had improved survival (HR=0.85; 95% CI: 0.76- 0.96), compared to patients without PCP contact, despite their older age. This suggests that the results are biased to the null and provide a more conservative estimate of the improved survival among patients with pre-diagnosis PCP contact.
Women who had at least 1 PCP visit in 12 months before diagnosis of mBC were more likely to see a medical oncologist compared to those with no PCP visits, and this translated into significant improvements in survival and a 15% lower risk of dying. We find a threshold effect whereby we only see a statistically significant positive association with a medical oncologist visit among individuals with 3 or more prior PCP visits, 3-4 visits-1.29 (1.11 - 1.50) p<0.01; 5 or more visits 1.41(1.22 - 1.63) p<0.0001. In terms of the medical oncologist visit, there were no differences between those with 1 or 2 PCP visits and those with zero PCP visits. Taken together, this suggests that it is not just a pre- diagnosis PCP visit but rather the intensity of PCP visits that impacts referral to the medical oncologist. Women in the older age groups (75-84 and 85+ years) were less likely to see a medical oncologist following diagnosis; the HRs (95% CI) were 0.84 (0.75 - 0.93) and 0.61 (0.52 - 0.70), respectively, compared to women aged 66-74 years. However, once post-diagnosis contact with medical oncology was established there was no further effect on mortality regardless of the number of post diagnosis PCP visits, suggesting that it is the post-diagnosis medical oncology care that primarily drives the improvements in mortality in this patient population. Previous studies have reported that PCPs play an important role in the early detection of breast cancer through cancer screening [13,14]. In addition, others have shown that increasing number of PCP visits in the period prior to BC diagnosis has been associated with improved BC-related outcomes, including lower odds of late-stage diagnosis, and lower BC-specific and overall mortality [15,16]; however, the impact of subsequent post referral medical oncology care was not evaluated in these studies.
A recent study by Park et al. [20] looking at a SEER sample of patients with all stages of breast cancer indicates that although overall age-adjusted breast cancer mortality rates decreased by almost onethird, from 33.5% in 1988 to 23.5% in 2010, these improvements were more evident in women younger than 70, and were attributed to treatment and not necessarily to earlier diagnosis. Therefore, prompt referral to medical oncology post diagnosis of mBC seems important given the results of our study.
We also note that in our study older women with ER negative breast cancer have two times greater mortality and ER unknown disease 62% increased mortality compared to patients with ER positive disease, while women with poorly differentiated tumors have a 39% increased mortality compared to patients without poorly differentiated tumors. HER2neu and Ki-67 variables were not included in our analysis because they were not recorded in the SEERMedicare database during the study time frame.
In this study, we examined if PCP contact prior to metastatic breast cancer diagnosis affects survival independently of or is in some measure dependent on subsequent medical oncologist contact. Treatment receipt after diagnosis (including surgery, adjuvant systemic therapy, and radiation therapy) are factors in the causal pathway between the pre-diagnosis PCP contact and the outcome (survival), and may not be independently associated with the outcome; therefore, these factors were not included as potential confounders in our analysis.
Our results indicate that the probability of medical oncologist visit was similar among those with 0-2 PCP visits within 1 year of diagnosis, while more frequent (3+) PCP visits pre-diagnosis had positive impact on the probability of a post-diagnosis medical oncologist visit. Seventy seven percent (N=1,595) of women in the study cohort had at least 1 medical oncologist visit post diagnosis of mBC and these women had significantly lower mortality compared to those without any medical oncologist contact (HR=0.33; 95% CI: 0.29-0.38). However, this magnitude of reduction in mortality risk is potentially subject to selection bias, for instance, it may be that healthier patients saw medical oncologists and were able to receive anti-cancer treatments. In addition, we found that several other factors and not medical insurance per se, influenced the referral patterns to a medical oncologist in this Medicare healthcare funded population. Among these factors were older age, African American race and lower socioeconomic status, all of which significantly decreased referral to medical oncology.
Potential barriers to patient referral to specialty care have been reported by others, and include patient factors such as financial and socioeconomic constraints, and system factors such as restricted provider networks and preauthorization requirements [12]. In addition to these factors, an individual’s ability to navigate and engage in the healthcare system during the pre-diagnosis period, as at least partly evidenced by regular contact with a PCP, could also influence referral to specialty care following their cancer diagnosis.
Our observations indicate that more frequent pre-cancer diagnosis PCP contact reduced all-cause mortality risk in the full sample of Medicare recipients with mBC but not in the subgroup of patients with at least 1 medical oncologist visit after diagnosis of mBC. Given that the PCP contact before the diagnosis of mBC does not have an independent additive effect on survival; it is plausible that the PCP contact pre diagnosis and subsequent referral to medical oncology likely initiated by the PCP has a crucial positive impact on the overall survival of older patients with mBC. One of the potential study limitations, however, is the possibility of misclassification or not being able to fully capture some specialist visits due to missing specialty information; this may explain why some women were found to have no PCP and no medical oncologist visits.
In addition, although our results highlight the importance and positive impact of the PCP and the medical oncologist care on the survival outcomes of older mBC patients, they may not necessarily be generalizable to non-Medicare beneficiaries or younger women with mBC.
In summary, more frequent pre-diagnosis primary care contact impacts positively referral patterns to medical oncology with subsequent benefits of oncology focused breast cancer care. The enhanced access to medical oncology subspecialty care after diagnosis of mBC is associated with improved all cause mortality in Medicare recipients with incident mBC.
Acknowledgement
This study was funded by Amgen, Inc. The authors wish to thank Pharmaceutical Research Computing for programming assistance on the primary datasets. This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the Applied Research Program, NCI; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEERMedicare database. The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35136 awarded to the Northern California Cancer Center, contract N01-PC-35139 awarded to the University of Southern California, and contract N02- PC-15105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #U55/CCR921930-02 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the author(s) and endorsement by the State of California, Department of Public Health the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors is not intended nor should be inferred. Part of A.H.’s time was supported by a Merit Review Award (I01 BX000545), Department of Veterans Affairs.
References
- Gennari A, Conte P, Rosso R, Orlandini C, Bruzzi P. Survival of metastatic breast carcinoma patients over a 20-year period: a retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005; 104: 1742-1750.
- Largillier R, Ferrero JM, Doyen J, Barriere J, Namer M, Mari V, et al. Prognostic factors in 1,038 women with metastatic breast cancer. Ann Oncol. 2008; 19: 2012-2019.
- Foukakis T, Fornander T, Lekberg T, Hellborg H, Adolfsson J, Bergh J. Agespecific trends of survival in metastatic breast cancer: 26 years longitudinal data from a population-based cancer registry in Stockholm, Sweden. Breast Cancer Res Treat. 2011; 130: 553-560.
- Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, et al. ESOESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Ann Oncol. 2014; 25: 1871-1888.
- Chirgwin J, Craike M, Gray C, Watty K, Mileshkin L, Livingston PM. Does multidisciplinary care enhance the management of advanced breast cancer? evaluation of advanced breast cancer multidisciplinary team meetings. J Oncol Pract. 2010; 6: 294-300.
- Saini KS, Taylor C, Ramirez AJ, Palmieri C, Gunnarsson U, Schmoll HJ, et al. Role of the multidisciplinary team in breast cancer management: results from a large international survey involving 39 countries. Ann Oncol. 2012; 23: 853-859.
- Baena-Canada JM, Ramirez-Daffos P, Cortes-Carmona C, Rosado-Varela P, Nieto-Vera J, Benitez-Rodriguez E. Follow-up of long-term survivors of breast cancer in primary care versus specialist attention. Fam Pract. 2013; 30: 525-532.
- Luo R, Giordano SH, Freeman JL, Zhang D, Goodwin JS. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006; 11: 1025-1033.
- Davidoff AJ, Rapp T, Onukwugha E, Zuckerman IH, Hanna N, Pandya N, et al. Trends in disparities in receipt of adjuvant therapy for elderly stage III colon cancer patients: the role of the medical oncologist evaluation. Med Care. 2009; 47: 1229-1236.
- Hillner BE, Smith TJ, Desch CE. Hospital and physician volume or specialization and outcomes in cancer treatment: importance in quality of cancer care. J Clin Oncol. 2000; 18: 2327-2340.
- Baldwin LM, Dobie SA, Billingsley K, Cai Y, Wright GE, Dominitz JA, et al. Explaining black-white differences in receipt of recommended colon cancer treatment. J Natl Cancer Inst. 2005; 97: 1211-1220.
- Kwon DH, Tisnado DM, Keating NL, Klabunde CN, Adams JL, Rastegar A, et al. Physician-reported barriers to referring cancer patients to specialists: prevalence, factors, and association with career satisfaction. Cancer. 2015; 121: 113-122.
- Phillips KA, Haas JS, Liang SY, Baker LC, Tye S, Kerlikowske K, et al. Are gatekeeper requirements associated with cancer screening utilization? Health Serv Res. 2004; 39: 153-178.
- Haggstrom DA, Phillips KA, Liang SY, Haas JS, Tye S, Kerlikowske K. Variation in screening mammography and Papanicolaou smear by primary care physician specialty and gatekeeper plan (United States). Cancer Causes Control. 2004; 15: 883-892.
- Roetzheim RG, Ferrante JM, Lee JH, Chen R, Love-Jackson KM, Gonzalez EC, et al. Influence of primary care on breast cancer outcomes among Medicare beneficiaries. Ann Fam Med. 2012; 10: 401-411.
- Fisher KJ, Lee JH, Ferrante JM, McCarthy EP, Gonzalez EC, Chen R, et al. The effects of primary care on breast cancer mortality and incidence among Medicare beneficiaries. Cancer. 2013; 119: 2964-2972.
- Klabunde CN, Ambs A, Keating NL, He Y, Doucette WR, Tisnado D, et al. The role of primary care physicians in cancer care. J Gen Intern Med. 2009; 24: 1029-1036.
- Lippincott. AJCC. Manual for Staging of Cancer. 6th ed. 2002.
- Warren JL, Butler EN, Stevens J, Lathan CS, Noone AM, Ward KC, et al. Receipt of chemotherapy among medicare patients with cancer by type of supplemental insurance. J Clin Oncol. 2015; 33: 312-318.
- Park JH, Anderson WF, Gail MH. Improvements in US Breast Cancer Survival and Proportion Explained by Tumor Size and Estrogen-Receptor Status. J Clin Oncol. 2015; 33: 2870-2876.