
Special Article - Fall Prevention
Gerontol Geriatr Res. 2021; 7(2): 1056.
Added Sugar Intake and Risk of Falling Among Older People: The Seniors-ENRICA Cohort Study
Ballesteros JM1*, Struijk E1, Machado-Fragua MD1, Ortolá R1, Rodriguez-Artalejo F1,2, Lopez-Garcia E1,2
1Department of Preventive Medicine and Public Health, School of Medicine, Universidad Autónoma de Madrid-IdiPaz and CIBERESP (CIBER of Epidemiology and Public Health), Madrid, Spain
2IMDEA-Food Institute, CEI UAM+CSIC, Madrid, Spain
*Corresponding author: Ballesteros JM, Department of Preventive Medicine and Public Health, School of Medicine, Universidad Autónoma de Madrid, C/ Arzobispo Morcillo, s/n, 28029 Madrid, Spain
Received: May 11, 2021; Accepted: June 08, 2021; Published: June 15, 2021
Abstract
Background: Added sugar intake is a key contributor to the development of several chronic diseases. We aimed to investigate the prospective association between added sugar intake and the risk of falling among older men and women.
Methods: We analyzed data from 2,154 Spanish adults aged ≥65 years from the Seniors-ENRICA cohort. Baseline food consumption was collected in 2008-2010 with a validated diet history, in which 155 foods were identified to contain added sugar. The occurrence of falls was ascertained up to 2015. Analyses were conducted with Cox models adjusted for potential confounders, including nutritional status, chronic diseases and sleeping medication.
Results: Over 7.2y of follow-up, 605 participants experienced ≥1 fall and 527 suffered injurious falls. The hazard ratios (95% confidence interval) for ≥1 fall across quintiles of added sugar intake were: 1.0, 1.09 (0.83-1.42), 1.07 (0.82-1.40), 1.15 (0.88-1.52), and 1.48 (1.12-1.96); p-trend 0.03. The corresponding figures for injurious falls were: 1.0, 1.17 (0.88-1.56), 1.06 (0.79- 1.41), 1.13 (0.84-1.52), and 1.40 (1.03-1.90); p-trend 0.10. These associations did not vary over strata of age, protein, calcium or vitamin intake, diet quality, physical activity or alcohol consumption. No differences were found when solid and liquid sources of added sugars were examined separately.
Conclusions: Intake of added sugars was associated with a higher risk of falling in older people. This adds to the evidence to support interventions to reduce added sugar intake.
Keywords: Added sugars; Falls; Elderly; Cohort study
Introduction
Added sugar intake is a key contributor to the development of the most prevalent chronic diseases, including cardiovascular disease [1], type 2 diabetes [2], hypertension [3], and obesity [4]. Recent studies have also found an association between sugary drink consumption and risk of cancer [5], and between added sugar intake and frailty risk [6]. Accordingly, the World Health Organization has recommended to reduce the daily intake of free sugars below 10% of the total energy intake, and called for a further reduction below 5% to provide additional health benefits [7]. Moreover, according to the American Heart Association, women should consume <100 kcal/d and men <150kcal/d from added sugars [8]. However, current consumption of added sugars represents an average of 7.3% of the total energy intake per day in Spain [9] and 14.9% in the United States [1].
About one-third of people over 65 years of age fall each year; this percentage increases to 50% among those over 80 years or who live in nursing homes [10,11]. Falls in older people are associated with many adverse health outcomes; specifically, a substantial proportion of falls results in serious injuries (28% of them lead to fractures) and disability [12]. Also the “post-fall syndrome” is of relevance because it produces loss of self-confidence and autonomy, social withdrawal and physical decline that, in turn, increases the risk of subsequent falls [13,14]. The high incidence of falls in older adults and their long-term consequences represent a significant burden for healthcare systems, and compromise the sustainability of social protection systems in many countries [15,16].
Unfortunately, knowledge of the effect of diet on the risk of falling is still very limited. Poor nutritional status has been associated with an increased risk of falling [17] while adherence to a Mediterranean-style diet has been linked to lower likelihood of falls in older people [18]. However, studies that focused on specific nutrients, such as protein [19], calcium and vitamin D [20], or foods like fruit and vegetables [21] had inconclusive results. To the best of our knowledge, there is no information about the role of added sugars in this process. Therefore, we aimed to investigate the association between added sugar intake and the risk of falling in a cohort of community-dwelling older adults in Spain.
Methods
Study population
Data were taken from people participating in the Seniors-ENRICA (Study on Nutrition and Cardiovascular Risk in Spain) cohort study [22]. Baseline information was collected between 2008 and 2010 in three stages: first, a phone interview to obtain data on health status, lifestyle, morbidity, and health services use; second, a home visit to collect blood and urine samples; and finally, a second home visit to perform a physical examination and to record usual diet and prescribed medication. Follow-up data collection was conducted in 2012 and 2015, following the same protocols than in baseline. Participants were followed up to the date of the occurrence of the outcome, death, moment in which they abandoned the study, or the end of the study in 2017, whichever came first. The study was approved by the Clinical Research Ethics Committee of La Paz University Hospital in Madrid, and informed written consent was provided by all the participants.
From the 2,452 participant’s ≥65 years at baseline, we excluded 13 with implausible energy intake (outside the range of 800-5,000 kcal/d for men and 500-4,000 kcal/d for women). We also excluded 285 individuals who were frail, defined as having ≥3 criteria in the FRAIL scale [23], or lacked data on this variable at baseline, because frailty is a strong predictor of falls. Therefore, the analyses were conducted with 2,154 individuals.
Study variables
Food consumption: Baseline food consumption was obtained with a validated computer-assisted face-to-face dietary history, which was developed from that used in the EPIC-cohort study in Spain [24]. Study participants reported their weekly consumption of 861 different foods that were classified according to their nutritional content. We considered as added sugars those that were artificially present in foods (mainly mono- and di-saccharides added due to their sweetening properties), by contrast to those naturally existing in fruits, vegetables, and milk. We selected 155 foods containing added sugars, which were classified in the following food groups: table sugar, honey and syrups, special breads, baked goods and cookies, pastries, breakfast cereals, flavored milk, yogurt and fermented milk, dairy desserts, sweetened cheeses, canned fruits, jam and jelly, candy, chocolate, soft drinks, juice and nectars. Intake of sugar, macronutrients and energy were calculated using standard food composition tables [25-28] and were adjusted for energy intake using the residual method [29]. The validity of the diet history has previously been assessed by comparison against seven 24-hour recalls during one year [30]. Pearson’s correlation coefficients for the main food groups were the following: cereals (r= 0.66), meat (r= 0.66), fish (r= 0.42), vegetables (r= 0.62) and fruits (r= 0.44); and for the main nutrients were: total protein (r= 0.58), fats (r= 0.73), total carbohydrates (r= 0.66), fiber (r= 0.49) and total sugars (r= 0.55). Regarding the reliability of the diet history, the intraclass correlation coefficient between two diet assessments was >0.40 for most foods and nutrients [30].
Falls: Incident falls were obtained in 2012 and 2015 by asking the participants how many times they had fallen since the last interview. The responses were recorded from zero (no falls) to nine or more falls. Due to the small number of participants with recurrent falls, we classified the main outcome of this study into two categories: no falls and ≥1 fall. A secondary outcome was the consequences of falls. People who had fallen also reported if, as a result, they had injuries (including contusion, bruise, wounds, shock, unconsciousness, or fracture) or needed healthcare assistance (including consultation with a doctor or nurse, emergency care or hospital admission).
Potential confounders: Socio-demographic data recorded at baseline included sex, age and education (primary, secondary, and university studies). Tobacco consumption was categorized as never, former, and current smoking. Physical activity during leisure time was assessed with the EPIC-cohort questionnaire validated in Spain [31], and was expressed in tertiles of metabolic equivalent taskshour/ week. Weight and height were measured under standardized conditions; the Body Mass Index (BMI) was calculated as weight (kg) divided by squared height (m) and classified as <25, 25-29.9, and ≥30 kg/m². Alcohol consumption was categorized into never drinking, former drinking, moderate intake, and excessive intake (the threshold between moderate and excessive intake was 24g/day in women and 40g/day in men). Total energy, protein, vegetables, calcium and vitamin D intake were categorized in tertiles. The MEDAS score was used to assess accordance with the Mediterranean diet pattern, an indicator of diet quality [32]. Use of sleeping pills was self-reported and checked against drug packages at home and categorized as regular or occasional. Study participants also reported the following physician-diagnosed diseases: cardiovascular disease (ischemic heart disease, stroke, and heart failure), type 2 diabetes, cancer, hypertension, asthma or chronic bronchitis, osteomuscular disease (osteoarthritis, arthritis and hip fracture), and depression needing drug treatment.
Statistical analyses
Differences in sociodemographic, lifestyle and clinical variables across quintiles of added sugar intake were assessed with the chi-square test for qualitative variables and the ANOVA test for continuous variables. Person-years of follow-up were calculated from the date of the baseline questionnaire (starting in March 2008) until the date of the outcome, death, loss to follow-up, or the end of the study (June 2015), whichever came first. Cox proportional hazard models were used to estimate the Hazard Ratios (HR) and their 95% Confidence Interval (CI) for the association between added sugar intake and the incidence of falls (≥1 fall, injurious falls, and falls requiring healthcare assistance). Three sequential multivariable models were fitted: the first one was adjusted for sex, age, and education; the second was further adjusted for lifestyle (smoking status, BMI, physical activity, energy, protein, calcium and vitamin D intake, and alcohol consumption); and the third model was additionally adjusted for chronic diseases and sleeping medication to understand their impact on the studied association. To investigate the linear dose-response relation, we modeled the quintiles of added sugar intake as a continuous variable.
In sensitivity analysis we replicated the Cox models using as exposure the cumulative average of added sugar intake from questionnaires in years 2008-10 to 2013. We also performed analysis stratified by age (<70 vs. ≥70 years), protein intake (<60.6, which is the minimum protein requirement recommended for elderly people by the WHO, according to their weight [33] vs. ≥60.6g/d), the median of vegetables, calcium and vitamin D intake, MEDAS score, and physical activity, and also by alcohol consumption (drinkers vs. nondrinkers). Due to the limited sample size, these stratified analyses were based on tertiles of added sugar.
In an attempt to understand variations of the effect of added sugars depending on the food source, we analyzed the risk of falling by comparing the lowest against the highest tertile of the intake of sugary food groups more frequently consumed by the participants in the study: table sugar and candy, yogurt and fermented milk, pastries and cookies, soft drinks, juice and nectars. Finally, we also investigated the studied association by gathering together solid and liquid sources of added sugars. Statistical analyses were performed with STATA, version 15.0. This manuscript follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) recommendations. The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Results
In our cohort, mean consumption of added sugars was 19.5 (SD 21) g/d, representing 3.9% of the total energy intake. One-third of the added sugars intake was in the form of table sugar, honey and jam. The remaining two-thirds were sugars added during food processing, mainly in pastries and cookies, yogurt and fermented milk, soft drinks, juice and nectars. Also, 83.9% of participants did not drink soft drinks and 92.8% did not consume commercial juice or nectars.
Baseline characteristics of the study participants across quintiles of daily intake of added sugars are presented in Table 1. Those in the highest versus the lowest quintile of intake were more frequently women, had less physical activity, and a lower frequency of diabetes and excessive alcohol intake. Total energy intake increased across the quintiles of added sugars while the quality of the diet (MEDAS score) decreased. Protein, calcium, and vitamin D intakes were higher among participants in the highest quintile.
Added sugar intake, quintiles of g/d
Overall
Q1 0-<2.3
Q3 =9.2 – <18.5
Q5 =33.8
p*
n
2154
431
431
430
Added sugar, g/d
19.5 (21)
0.4 (0.7)
13.5 (2.7)
52.5 (20)
<0.001
Added sugar, % E
3.9 (3.9)
0.1 (0.2)
3.0 (1.1)
9.7 (4.1)
<0.001
Sex, women, %
55.3
47.3
54.5
59.1
0.002
Age, years
71.7 (5.7)
71.4 (5.6)
72.0 (5.9)
71.9 (5.6)
0.4
Educational level, %
0.6
=Primary
61.6
61.9
61
60.9
Secondary
20.2
17.8
21.3
22.6
University
18.1
20.9
17.6
16.5
Tobacco consumption, %
0.83
Never smoker
61
58
61.7
62.8
Former smoker
29
32.3
27.8
28.1
Current smoker
10.1
9.7
10.4
9.1
Physical activity, METs-h/week
21.2 (15)
22.4 (16)
22.0 (15)
20.6 (15)
<0.001
BMI, kg/m2, %
0.37
<25
19.3
20.7
17.8
21.5
25-29.9
48.2
48
46
48.5
=30
32.5
31.3
36.2
30
Alcohol consumption, %
<0.001
Never drinker
37.2
36.6
37.2
38.4
Former drinker
9.4
7
9.4
15.1
Moderate alcohol intake1
46.2
46
47.8
41.7
Excessive alcohol intake 1
7.2
10.3
5.6
4.8
Energy intake, kcal/d
1986 (706)
1822 (885)
1941 (531)
2291 (683)
<0.001
Protein intake, g/d
89 (27)
85 (28)
90 (25)
93 (30)
<0.001
Vegetables intake, g/d
207 (136)
203 (138)
213 (142)
204 (131)
0.55
Calcium intake, mg/d
879 (325)
793 (352)
894 (311)
939 (308)
0.03
Vitamin D intake, μg/d
3.3 (3.0)
3.3 (3.9)
3.4 (2.6)
3.4 (3.0)
<0.001
MEDAS
7.1 (1.8)
7.8 (1.6)
7.3 (1.7)
6.1 (2.0)
<0.001
Sleeping pills regularly, %
15.6
13.5
18.3
14.2
0.27
Diagnosed diseases, %
Cardiovascular disease2
6
6.3
5.6
6.7
0.32
Diabetes
17.5
21.3
18.3
14.2
0.03
Cancer
2.3
2.3
2.5
3.3
0.48
Hypertension
66.9
68.9
66.6
67
0.42
Asthma or chronic bronchitis
7.3
6
6.5
6.8
0.24
Osteo-muscular diseases3
52
50.3
52.2
50.2
0.69
Depression needing treatment
8.1
7.4
8.1
9.3
0.84
Continuous variables are given as means (SD). *P-values based on chi-square test for qualitative variables or ANOVA test for continuous variables.
1Moderate intake: <24g/d alcohol in women, <40g/d in men. Excessive intake: =24g/d in women, =40g/d in men.
2Includes ischemic heart disease, stroke and heart failure.
3Includes osteo-arthritis, arthritis and hip fracture.
Table 1: Baseline characteristics of study participants across quintiles of daily intake of added sugars (n=2,154).
Over 7.2 y of follow-up, 605 participants (28%) reported ≥1 fall (Table 2). We found a dose-response association between added sugar intake and risk of ≥1 fall in semi-crude models, which remained significant after full adjustment for potential confounders; fullyadjusted HR (95% CI) across quintiles of intake were 1.0, 1.09 (0.83- 1.42), 1.07 (0.82-1.40), 1.15 (0.88-1.52), and 1.48 (1.12-1.96), p-trend 0.03. Corresponding figures for injurious falls were 1.0, 1.17 (0.88- 1.56), 1.06 (0.79-1.41), 1.13 (0.84-1.52), and 1.40 (1.03-1.90), p-trend 0.10; and HR for falls requiring healthcare were 1.0, 1.18 (0.80-1.73), 1.01 (0.68-1.49), 1.31 (0.89-1.91), and 1.22 (0.81-1.83), p-trend 0.83.
Added sugar intake, quintiles of g/d
Q1 Range: 0 - <2.3
Q2 =2.3 - <9.2
Q3 =9.2 - <18.5
Q4 =18.5 - <33.8
Q5 =33.8
P for trend
Participants, n
431
431
431
431
430
Risk of =1 fall
Person-yr/cases
2203/112
2114/118
2125/118
2101/123
2046/134
Model 1
1
1.08 (0.83-1.40)
1.06 (0.81-1.37)
1.17 (0.91-1.52)
1.36 (1.05-1.75)
0.03
Model 2
1
1.10 (0.84-1.44)
1.09 (0.83-1.43)
1.17 (0.89-1.53)
1.46 (1.10-1.93)
0.03
Model 3
1
1.09 (0.83-1.42)
1.07 (0.82-1.40)
1.15 (0.88-1.52)
1.48 (1.12-1.96)
0.03
Risk of injurious falls1
Person-yr/cases
2223/98
2122/108
2138/101
2117/106
2065/114
Model 1
1
1.14 (0.87-1.50)
1.04 (0.79-1.38)
1.15 (0.87-1.52)
1.30 (0.99-1.71)
0.09
Model 2
1
1.19 (0.89-1.57)
1.08 (0.80-1.44)
1.14 (0.85-1.52)
1.39 (1.02-1.87)
0.1
Model 3
1
1.17 (0.88-1.56)
1.06 (0.79-1.41)
1.13 (0.84-1.52)
1.40 (1.03-1.90)
0.1
Risk of falls that required healthcare2
Person-yr/cases
2297/52
2178/60
2211/59
2184/71
2139/64
Model 1
1
1.14 (0.79-1.66)
1.04 (0.71-1.52)
1.37 (0.96-1.97)
1.27 (0.88-1.83)
0.45
Model 2
1
1.24 (0.85-1.82)
1.05 (0.71-1.54)
1.35 (0.93-1.96)
1.25 (0.84-1.88)
0.79
Model 3
1
1.18 (0.80-1.73)
1.01 (0.68-1.49)
1.31 (0.89-1.91)
1.22 (0.81-1.83)
0.83
Model 1: Cox regression model adjusted for sex, age, and educational level (=primary, secondary, university).
Model 2: Additionally adjusted for tobacco (never smoker, former smoker, current smoker), physical activity (tertiles of METs-h/wk), BMI (<25, 25-29.9, =30 kg/m2), alcohol consumption (never drinker, former drinker, moderate intake, excessive intake), energy intake (tertiles of kcal/d), protein intake (tertiles g/d), calcium intake (tertiles mg/d), and vitamin D intake (tertiles μg/d).
Model 3: Additionally adjusted for regular use of sleeping pills, cardiovascular disease, type 2 diabetes, cancer, hypertension, osteo-muscular disease, asthma or chronic bronchitis, and depression requiring treatment.
1Falls with contusion, bruise, wounds, shock, unconsciousness, or fracture.
2Falls that had required consultation with a doctor or nurse, emergency care, or hospital admission.
Table 2: Hazard ratios (95% confidence interval) for the association between added sugar intake and risk of ≥1 fall, injurious falls, and falls that required healthcare (n=2,154).
In stratified analysis by tertiles of added sugars (Figure 1) no differences were observed between categories of age, protein, calcium or vitamin intake, diet quality, physical activity or alcohol consumption. The analysis on cumulative added sugar intake (Supplemental Table 1) showed the same tendency to an increased risk of falling, having injurious falls, and falls requiring healthcare when compared the highest against the lowest quintile. The difference between extreme quintiles (q) of cumulative intake was smaller than the difference between extreme quintiles of the baseline exposure (mean accumulated exposure, q5: 42.5g/d vs. q1:4.6g/d; mean baseline exposure, q5: 52.5g/d vs. q1: 0.4g/d).
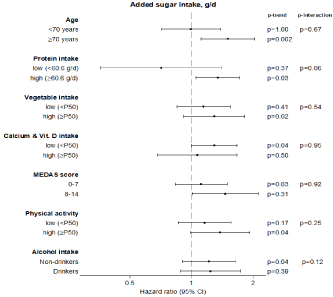
Figure 1: Hazard ratios (95% confidence interval)* for the risk of ≥1 falls among participants in the highest versus the lowest tertile of added sugar intake, stratified
by age, protein intake, vegetables consumption, calcium and vitamin D intake, MEDAS score, physical activity and alcohol intake.
Footnote: Cox regression model adjusted for sex, age, educational level (≤primary, secondary, university), tobacco (never smoker, former smoker, current smoker),
physical activity (tertiles of METs-h/wk), BMI (<25, 25-29.9, ≥30 kg/m²), alcohol consumption (never drinker, former drinker, moderate intake, excessive intake),
energy intake (tertiles of kcal/d), protein intake (tertiles g/d), calcium intake (tertiles mg/d), and vitamin D intake (tertiles μg/d), regular use of sleeping pills,
cardiovascular disease, type 2 diabetes, cancer, hypertension, osteo-muscular disease, asthma or chronic bronchitis, and depression requiring treatment, except
for the stratification variable.
*The lines depict the 95% Confidence Interval (CI).
Finally, when we studied the effect of individual food sources of added sugars (Supplemental Table 2) we found a significant association for table sugar and candy (HR (95% CI) highest vs. lowest tertile: 1.32 (1.06-1.63), p-trend 0.01), but not for other food sources of sugar. Intake of liquid foods (soft drinks, juice, and nectars) was very heterogeneous which explains the gaps in the second tertile. Liquid food sources of added sugars showed a strong tendency to a higher risk of ≥1 falls (HR (95% CI) highest vs. lowest tertile: 1.23 (1.02-1.48), p-trend 0.08), while solid sources of added sugars showed some tendency to a higher risk (HR (95% CI) highest vs. lowest tertile: 1.17 (0.94-1.459, p-trend 0.13).
Discussion
In this study, a higher intake of added sugars was associated with an increased risk of experiencing ≥1 fall and injurious falls. This association was also statistically significant when sugars came only from table sugar and candy, but not from other individual sugary food sources, probably due to the low consumption of those foods. Solid and liquid sources of added sugars showed a similar tendency to a higher risk of falling.
The mean intake of added sugars (3.9% of total energy) was slightly lower than in a previous study among older people in Spain (5.1%) [9] In addition, the mean % energy intake from added sugars and the number of participants with at least 10% energy from added sugars (7.3%) were lower than in the United States (13.6% energy and 65.4% participants ≥10% energy in those ≥60 years in NHANES 2005-10) [1] Since our results indicate that even relatively low intakes of added sugars (the mean added sugar intake in the highest quintile was 9.7% of total energy) are associated with higher falls risk, the detrimental impact of this intake may be considerable in populations with higher added sugar intake.
In recent years, evidence has emerged on the adverse health effects of a high intake of added sugars. Observational studies have consistently shown that added sugars increase the risk of many chronic diseases and frailty [1-6] that, in turn, are risk factors of falling. The mechanisms are only partially understood. For example, in some population studies [34,35], those with a high intake of added sugars showed greater energy intake and less adherence to healthy dietary patterns. However, in our study, the association between added sugars and falls remained significant in participants with sufficient protein intake or with better adherence to the Mediterranean diet. Another mechanism could be the low-grade inflammation and insulin resistance resulting from high added sugar intake, which contributes to the pathogenesis of sarcopenia, an important risk factor of falls [36,37]. In addition, hyperglycemia and hyperinsulinemia can alter bone remodeling through the accumulation of products coming from the reaction between sugars and free amino groups in proteins, modifying the microarchitecture and bone tissue properties [38]. Finally, consumption of sugar-sweetened beverages has been associated with higher risk of diabetes [2], and older people with diabetic peripheral neuropathy have impaired ability to stabilize their body when walking on irregular surfaces [39]. Therefore, it seems that a set of biological pathways, rather than a single mechanism, could contribute to explain the association between added sugar intake and risk of falling.
We are not aware of previous studies on the association between added sugar and risk of falling in older people. However, in a previous analysis with the Seniors-ENRICA cohort [6], participants in the highest vs. lowest tertile of added sugar intake had increased odds of frailty; foods whose sugar was added during manufacturing (e.g. pastries and cookies) showed the strongest association with frailty. Likewise, among 73,572 postmenopausal women from the Nurse’s Health Study cohort [40], soda consumption was associated with an increased risk of hip fracture, a falls-related outcome. In that study, an excess risk was found in consumers of both regular and diet soda and did not differ between colas and non-cola soft drinks. In our analysis, there is a strong tendency to a higher risk of falling in the association with added sugar intake from liquid food sources (HR (95%CI) highest vs. lowest tertile: 1.23 (1.02-1.48), p-trend 0.08), along the same line as other studies [41,42]. However, we did not find a separate association for soft drinks, possible due to the low consumption in our cohort, particularly when compared to other populations. The smaller difference between extreme quintiles (q) of cumulative intake when compared to quintiles of baseline intake (mean accumulated exposure, q5: 42.5g/d vs. q1:4.6g/d; mean baseline exposure, q5: 52.5g/d vs. q1: 0.4g/d) may explain the absence of statistically significance for each estimate across categories of cumulative intake.
There seemed to be a strong tendency to a higher risk of injurious falling among participants in the highest tertile: HR (95%CI) 1.40 (1.02-1.91), although the p for trend was nonsignificant, while there is some tendency to higher risk of falls requiring healthcare. It is possible that statistical significance was not reached due to the small number of cases. Indeed, a limitation of this study is the relatively modest number of outcomes that may have limited statistical power, particularly in stratified analysis. Another limitation of our study is that incident falls were self-reported, so some events could have been missed because of recall problems. Cummings et al. showed in a 12-month prospective study of 304 ambulatory adults over the age of 60 that, depending on the time period of recall, 13-32% of those with confirmed falls did not recall falling during that time [43]. Thus, although we tried to minimize this limitation using trained staff to conduct the interviews, it may have resulted in underestimation of the study association. Finally, as in any observational study and despite adjustment for a good number of covariates, some residual confounding may persist. Regardless of these limitations, to our knowledge no previous study has investigated the association between added sugar intake and risk of falling in the elderly. Other strengths of the present study are its prospective design, a detailed assessment of food consumption with a validated diet history, and a long follow-up.
Conclusion
In conclusion, the intake of added sugars was associated with a higher risk of falling in older adults. Future research should confirm these findings in other populations. Our results are in line with the existing evidence on the adverse health impact of added sugars. This, together with the overconsumption of sugars worldwide, should support the implementation of measures to reduce added sugars intake.
Sources of Support
This work was supported by FIS grants 16/609 and 16/1512 (Instituto de Salud Carlos III, State Secretary of R+D+I, and FEDER/FSE), the ATHLOS project (EU H2020- Project ID: 635316) and the JPI HDHL (SALAMANDER project).
The funding agencies had no role in the study design, data analysis, interpretation of results, writing of the report, and in the decision to submit the article for publication.
Author’s Contributions
Ballesteros JM and Lopez-Garcia E designed the research. Ballesteros JM performed the statistical analyses. All authors contributed to results interpretation. Ballesteros JM, Rodríguez- Artalejo F and Lopez-Garcia E drafted the manuscript. Lopez-Garcia E supervised the conduct of research. Lopez-Garcia E had primary responsibility for final content. All authors reviewed the manuscript for important intellectual content, read and approved the final manuscript.
References
- Yang Q, Zhang Z, Gregg E, Dana Flanders W, Merritt R, Hu Fb. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014; 174: 516-524.
- Malik V, Popkin B, Gray G, Després J, Hu F. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010; 33: 2477-2483.
- Brown I, Stamler J, Van Horn L, Robertson C, Chan Q, Huang CC, et al. Sugar-sweetened beverage, sugar intake of individuals, and their blood pressure: international study of macro/micronutrients and blood pressure. Hypertension. 2011; 57: 695-701.
- Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analyses of randomized controlled trials and cohort studies. BMJ. 2012; 345: e7492.
- Chazelas E, Srour B, Desmetz E, Kesse-Guyot E, Julia C, Druesne-Pecollo N, et al. Sugary drink consumption and risk of cancer: results from NutriNet- Santé prospective cohort. BMJ. 2019; 366: l2408.
- Laclaustra M, Rodríguez-Artalejo F, Guallar-Castillón P, Banegas J, Graciani A, et al. Prospective association between added sugars and frailty in older adults. Am J Clin Nutr. 2018; 107: 772-779.
- World Health Organization. Sugars intake for adults and children: guidelines. Geneva: WHO. 2015.
- Johnson R, Appel L, Brands M, Howard B, Lefevre M, Sacks F, et al. Dietary Sugars Intake and Cardiovascular Health: A scientific statement from the American Heart Association. Circulation. 2009; 120: 1011-1020.
- Ruiz E, Rodríguez P, Valero T, Ávila JM, Aranceta-Bartrina J, González- Gross M, et al. Dietary intake of individual/free and intrinsic) sugars and food sources in the Spanish population: findings from the ANIBES study. Nutrients. 2017; 9: 275.
- Rubenstein L. Falls in older people: epidemiology, risk factors and strategies for prevention. Age Ageing. 2006; 35: ii37-ii41.
- Ambrose A, Paul G, Hausdorff J. Risk factors for falls among older adults: A review of the literature. Maturitas. 2013; 75: 51-61.
- Verma S, Willets J, Corns H, Marucci-Wellman HR, Lombardi DA, Courtney TK. Falls and fall-related injuries among community-dwelling adults in the United States. PLoS One. 2016; 11: e0150939.
- US Preventive Services Task Force Recommendation. Interventions to prevent fall in community-dwelling older adults. JAMA. 2018; 319: 1696-1704.
- Guirguis-Blake J, Michael Y, Perdue L, Coppola E, Beil T. Interventions to prevent falls in older adults. Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018; 319: 1705- 1716.
- Stevens J, Corso P, Finkelstein E, Miller T. The costs of fatal and non-fatal falls among older adults. Inj Prev. 2006; 12: 290-295.
- Burns ER, Lee R. The direct costs of fatal and non-fatal falls among older adults: United States. J Saf Res. 2016; 58: 99-103.
- Torres M, Féart C, Samieri C, Dorigny B, Luiking Y, Berr C, et al. Poor nutritional status is associated with a higher risk of falling and fracture in elderly people living at home in France: the Three-City cohort study. Osteoporos Int. 2015; 26: 2157-2164.
- Ballesteros J, Struijk E, Rodríguez-Artalejo F, Lopez-Garcia E. Mediterranean diet and risk of falling in community-dwelling older adults. Clinical Nutrition. 2019.
- Zoltick E, Sahni S, Mc Lean R, Quach L, Casey VA, Hannan MT. Dietary protein intake and subsequent falls in older men and women: the Framingham study. J Nutr Health Aging. 2011; 15: 147-152.
- Jackson C, Gaugris S, Sen S, Hosking D. The effect of cholecalciferol (vitamin D3) on the risk of fall and fracture: a meta-analysis. Q J Med. 2007; 100: 185-192.
- Luo SY, Li H, Luo H, Yin Xh, Lin du R, Zhao K, et al. Increased intake of vegetables, but not fruits, may be associated with reduced risk of hip fracture: a meta-analysis. Sci Rep. 2016; 6: 19783.
- Rodríguez-Artalejo F, Graciani A, Guallar-Castillón P, León-Muñoz L, Zuluaga M, Gutiérrez-Fisac JL, et al. Rationale and methods of the study on nutrition and cardiovascular risk in Spain (ENRICA). Rev Esp Cardiol. 2011; 64: 876-882.
- Morley J, Vellas B, Van Kan G, Anker S, Bauer J, Cesari M, et al. Frailty consensus: a call for action. J Am Med Dir Assoc. 2013; 14: 392-397.
- EPIC Group of Spain. European Prospective Investigation into Cancer and Nutrition. Relative validity and reproducibility of a diet history questionnaire in Spain. I. Foods Int J Epidemiol. 1997; 26: S91-S99.
- Farrán A, Zamora R, Cervera P. Tablas de composición de alimentos del CESNID, 2ª edición. Edicions Universitat de Barcelona ed. Barcelona: McGraw-Hill/Interamericana de España SAU. 2004.
- Moreiras O, Carvajal A, Cabrera L, Cuadrado C. Tablas de composición de alimentos. 11th ed. Madrid: Ediciones Pirámide. 2007.
- United States Department of Agriculture. Agricultural Research Service. USDA National Nutrient Database for Standard Reference. 2010.
- Jiménez Cruz A, Cervera Ral P, Bacardí Gascón M. Tabla de composición de alimentos Barcelona: Wander-Sandoz Nutrition. 1990.
- Willett WC. Nutritional epidemiology. Third Edition. New York, NY: Oxford University Press. 2012.
- Guallar-Castillón P, Sagardui-Villamor J, Balboa-Castillo T, Sala-Vila A, Ariza M, León-Muñoz LM, et al. Validity and reproducibility of a Spanish dietary history. PLoS One. 2014; 9: e86074.
- Pols M, Peters P, Ocke M, Slimani N, Bueno-de-Mesquita H. Estimation of reproducibility and relative validity of the questions included in the EPIC Physical activity Questionaire. Int J Epidemiol. 1997; 26: S181-S189.
- Schröder H, Fitó M, Estruch R, Martínez-González M, Corella D, Salas- Salvadó J, et al. A short screener is valid for assesing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011; 141: 1140- 1145.
- WHO technical report series, n° 935. Protein and amino acid requirements in human nutrition: report of a joint WHO/FAO/UNU expert consultation. Geneva: World Health Organization. 2007.
- Gibson S. Dietary sugars intake and micronutrient adequacy: a systematic review of the evidence. Nutr Res Rev. 2007; 20: 121-131.
- Moshtaghian H, Louie J, Charlton K, Probst Y, Gopinath B, Mitchell P, et al. Added sugar intake that exceeds current recommendations is associated with nutrient dilution in older Australians. Nutrition 2016; 32: 937-942.
- Barzilay J, Blaum C, Moore T, Xue Q, Hirsch C, et al. Insulin resistance and inflammation as precursors of frailty: The Cardiovascular Health Study. Arch Intern Med. 2007; 167: 635-641.
- Cleasby M, Jamieson P, Atherton P. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016; 229: R67-R81.
- Hunt H, Torres A, Palomino P, Marty E, Saiyed R, Cohn M, et al. Altered tissue composition, microarchitecture, and mechanical performance in cancellous bone from men with type 2 diabetes mellitus. JBMR. 2019.
- Menz H, Lord S, St George R, Fitzpatrick R. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004; 85: 245-252.
- Fung TT, Arasaratnam MH, Grodstein F, Katz JN, Rosner B, Willett WC, Feskanich D. Soda consumption and risk of hip fractures in postmenopausal women in the Nurses’ Health Study. Am J Clin Nutr. 2014; 100: 953-958.
- Newens K, Walton J. A review of sugar consumption from nationally representative dietary surveys across the world. J Hum Nutr Diet. 2016; 29: 225-240.
- Malik VS, Hu FB. Fructose and cardiometabolic health: what the evidence from sugar-sweetened beverages tells us. J Am Coll Cardiol. 2015; 66: 1615- 1624.
- Cummings SR, Nevitt MC, and Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. Journal of the American Geriatrics Society (JAGS). 1988; 36: 613-616.