
Research Article
Gerontol Geriatr Res. 2022; 8(2): 1077.
Factors Associated with Frailty Transitions among the Old-Old in Community: A Prospective Cohort Study
Li J¹, Zhu M¹, Zhao S² and Liu X¹*
¹Department of Geriatrics, Chinese Academy of Medical Sciences, Peking Union Medical College, Peking Union Medical College Hospital, China
²Department of Geriatrics, Yanyuan Rehabilitation Hospital, China
*Corresponding author: Xiaohong Liu, Department of Geriatrics, Chinese Academy of Medical Sciences, Peking Union Medical College, Peking Union Medical College Hospital, No. 1 Shuai fu yuan, Dong cheng District, Beijing, 100730, China
Received: September 12, 2022; Accepted: October 18, 2022; Published: October 25, 2022
Abstract
Background: The identification of older adults with different frailty transitions, especially the old-old, is beneficial for stratified management. However, the factors associated with frailty transition in the elderly have not been fully elucidated. This study aimed to explore frailty transitions and associated factors among older adults.
Methods: The participants were all from a prospective cohort study of older adults aged ≥75 years in a continuing care retirement community in Beijing, China. Frailty states were assessed using FRAIL at baseline and 1-year follow-up. The association between factors, including comprehensive geriatric assessment and laboratory indicators such as serum albumin and highsensitivity C-reactive protein (hsCRP), and frailty transitions were explored by binary logistic regression. The predicted value of the factors associated with frailty transitions was analyzed using the receiver operating characteristic curve (ROC), and the area under the ROC curve (AUC) was calculated.
Results: A total of 183 older adults (mean age: 83.9±4.4 years; females, 59%) completed the frailty state assessment at baseline and 1-year followup. After adjusting for age and sex, walking speed(odds ratio [OR], 0.01; 95%confidence interval [CI]: 0.002-0.12),timed up-and-go (TUG) test(OR:1.08, 95% CI: 1.02-1.15), short physical performance battery(OR:0.79, 95% CI :0.68-0.92), serum albumin (OR:0.78, 95% CI:0.64-0.94), and serum hsCRP (OR:1.21, 95% CI:1.00-1.47) were associated with worsening of the frailty state. Cognitive function (OR:6.73, 95% CI:1.15-39.19) was associated with improving the frailty state. ROC analysis showed that low walking speed (AUC:0.81), long TUG test time(AUC:0.77), low Short Physical Performance Battery (SPPB)score (AUC:0.75), low serum albumin (AUC:0.68), and high serum hsCRP(AUC:0.80) could predict the decline in frailty state. Good cognitive function (AUC: 0.69) predicted an improvement in the frailty state.
Conclusions: Frailty is dynamic. The frailty state of the old-old with poor physical function, low serum albumin, and high serum hsCRP was more likely to decline, but it was more likely to improve with good cognitive function. Walking speed, TUG test, SPPB, serum albumin, serum hsCRP, and cognitive function may predict frailty transitions among the old-old.
Keywords: Comprehensive geriatric assessment; Frailty; Frailty transition; Old-old
Introduction
Frailty is a state of cumulative decline in the functions of various physiological systems in the elderly, increasing vulnerability to poor homeostasis after a stress event [1-3]. Frailtyis associated with various adverse outcomes such as falls, disability, decreased quality of life, and mortality [4-7]. The prevalence of frailty among the elderly in community varies widely from 4.0% to 59.1% [8]. In China, the overall weighted prevalence of frailty was 9.9% according to the Comprehensive Geriatric Assessment-Frailty Index [9]. As global aging becomes increasingly prominent, frailty has become a challenge to the health of older adults. Early identification and intervention might reverse frailty and reduce adverse outcomes [10], consistent with the purpose of healthy aging.
Frailty is dynamic and partly reversible [3,11,12], which means that non-frail older adults may revert to frailty; similarly, frailty in older adults may improve their frailty state to a non-frailty state. Research among older adults from communities in Taiwan showed that the 5-year mortality risk differed among older adults with different frailty transitions within 1 year [13]. Mexican research has shown that males who transitioned to a worse frail state had a significantly higher risk of hospitalization than those who remained, as well as had a higher Medicare payment [14]. Thus, exploring the risk factors for frailty transition may provide a reference for identifying high-risk groups and developing intervention strategies. Differences in physical and psychological characteristics between the young-old and old-old have been recognized [15,16]. However, the factors associated with frailty transition in the elderly remain to be fully explored.
Materials and Methods
Study Design and Population
This prospective cohort study aimed to identify the old-old with different frailty transitions to provide evidence for the stratified management of older adults. Older adults aged ≥75 years in a continuing care retirement community named Taikang Yanyuanin Beijing, China were recruited. Convenience sampling was used for participant selection. All participants aged ≥75 years who participated in the annual physical examination from June to August 2018were followed up for 12 months after recruitment into the study. To avoid the impact of acute diseases on physical function assessment and ensure effective communication during frailty assessment, we applied the following exclusion criteria: severe cognitive impairment diagnosed by specialist physicians, acute conditions including acute infection, acute cerebrovascular disease, acute heart failure or myocardial infarction, pulmonary embolism, and acute abdominal disease. Among the 230 older adults who met the inclusion criteria, 183 consented to participate and completed the follow-up.
Data Collection
One trained geriatrician from PUMCH and one trained general practitioner from Taikang Yanyuan conducted the Comprehensive Geriatric Assessment (CGA) and other data collection at baseline (from July to September 2018) and at the 1-year follow-up (from July to September 2019). The CGA included Katz’s activities of daily living [17] and physical functions including grip strength, usual walking speed in 6 m [18], timed up-and-go (TUG) test [19], and short physical performance battery (SPPB) [20]. It also included Mini-Mental State Examination(MMSE, normal,>24) [21], Geriatric Depression Scale- 15(GDS-15,normal,<5) [22], Mini-Nutritional Assessment-Short Form (MNA-SF,normal, ≥12) [23], Charlson Comorbidity Index (CCI) [24], and poly pharmacy (number of drugs ≥5).Additionally, laboratory parameters including white blood cell count, hemoglobin, serum albumin, serum high-sensitivity C-reactive protein (hsCRP), and Erythrocyte Sedimentation Rate (ESR) were recorded during physical examination.
Frailty Assessment
Frailty was assessed using FRAIL both at baseline and at the end of follow-up [25]. FRAIL is a self-rating scale with five dimensions, including fatigue (Do you feel tired at least three or four days per week?), resistance (Can you climb one floor without assistance?), ambulation (Can you walk one block without assistance?), illness (Do you suffer from more than five diseases?), and loss of weight (Has your weight decreased by ≥ 4.5 kg or 5% of baseline in the previous 12 months?). Those with no positive responses were robust, where as participants with one or two positive responses were considered prefrail, and those with three or more positive responses were considered frail. Robust or pre-frail participants were categorized as non-frail. The possible changes in the frailty state included robustness to prefrail or frail, pre-frail to robust or frail, and frail to pre-frail or robust. Frailty state transitions were deemed stability if they changed from non-frail to non-frail or from frail to frail; worsening, from non-frail to frail; and improving, from frail to non-frail).
Statistical Analysis
The clinical characteristics of the participants at baseline were expressed as the mean (standard deviation) or median (interquartile range) for continuous variables and as percentages for categorical data. Continuous variables were compared using Student’s t-test or Mann– Whitney U test, whereas categorical variables were compared using Pearson’s chi-square test or Fisher’s exact probability method. The change in frailty state after 12 months was described using a Sankey diagram (https://www.highcharts.com.cn/demo/highcharts/sankeydiagram). No data were missing. Univariate and multivariate binary logistic regression analyses were performed to assess the association between baseline characteristics and frailty transition in the nonfrail and frail participants. Frailty transitions between baseline and follow-up were used as dependent variables. Considering the sample size and collinearity between factors, clinical factors with statistically significant differences in the univariate analysiswere analyzed using binary logistic regression after adjustment for age and sex. A Receiver Operator Characteristic (ROC) curve was used to analyze the predictive value of factors related to frailty transitions. The area under the receiver operator characteristics (AUC) was calculated. All statistical analyses were performed using SPSS (25.0 version, IBM, New York, USA) and R (3.5.3 version, R Core Team, Vienna, Austria). A two-sided value of < 5% was considered statistically significant.
Results
Baseline Participant Characteristics
A total of 230 participants aged ≥75 years were eligible for the study; however, 47 participants failed to complete the follow-up, of which 7moved away, 38 refused frailty assessment, and 2 died. Therefore, 183participants were included in the analysis. The mean age was 83.9 ± 4.4 years (range, 75–94 years); 59.0% were female; and 46 (25.1%) and 137(74.9%) were frail and non-frail at baseline, respectively. The clinic odemographic characteristics and baseline frailty states showed no difference between those who completed the follow-up and those who did no (Table 1).
Completed the follow-up
Failed to follow-up
P value
Age, x- (SD) , year
83.9(4.4)
84.1(4.7)
0.77
Sex, female, n (%)
108(59%)
25(53.2%)
0.47
Education ,high school or above, n(%)
180(98.4%)
47(100.0%)
1.00
BMI, x- (SD) , kg/m2
24.0(3.2)
24.8(4.2)
0.24
Frail, n(%)
46(25.1%)
12(25.5%)
0.96
Non-frail,n(%)
137(74.9%)
35(74.5%)
0.96
ADL, M (IQR), score
ADL<6, n(%)6(6,6)
26(14.2%)6(6,6)
9(19.6%)0.41
0.37Gripstrength, M (IQR), kg
20.7(17.5,24.5)
20.4(17.2,28.1)
0.97
Walking speed,x- (SD), m/s
0.77(0.29)
0.72(0.33)
0.35
TUG,M (IQR),s
13.3(10.2,18.6)
14.4(10.5,24.3)
0.14
SPPB, M (IQR), score
9(5,10)
8(3,11)
0.55
MMSE,M (IQR), score
MMSE score<24, n(%)28(25,29)
30(16.5%)27(25,28)
9(19.1%)0.10
0.6713(12,14)
24(13.1%)13(12,14)
10(21.3%)0.71
0.16GDS-15, M (IQR), score
GDS-15 score>5 , n(%)2(0,3)
18(9.8%)2(1,4)
5(10.6%)0.37
0.87Number of drugs, M(IQR)
5(2,7)
5(3,7)
0.92
Poly pharmacy, n(%)
106(57.9%)
25(53.2%)
0.56
CCI, M (IQR)
1(0,2)
1(0,1)
0.83
White blood cell count, M (IQR), 109/ L
5.5(4.5,6.6)
6.0(4.8,7.4)
0.17
Hemoglobin, M (IQR), g/ L
134.5(125.0,144.0)
137.5(131.1,150.8)
0.13
Albumin, M (IQR), g/ L
42.1(40.2,44.5)
42.1(40.0,44.0)
0.71
HsCRP, M (IQR),mg/L
1.72(1.0,3.4)
2.02(1.0,3.6)
0.69
ESR, M (IQR),mm/h
15.5(10.0,26.1)
16.5(7.3,22.4)
0.77
Table 1: Comparison of baseline characteristics between participants who did and did not complete follow-up.
Frailty Transitions after 1-Year Follow-Up
An analysis of the FRAIL assessment results showed that whether the baseline frailty state was robust, pre-frail, or frail, state changes occurred between adjacent states (Figure 1). At the end of the followup period, most participants remained in the same frailty state or transitioned to a worse frailty state.
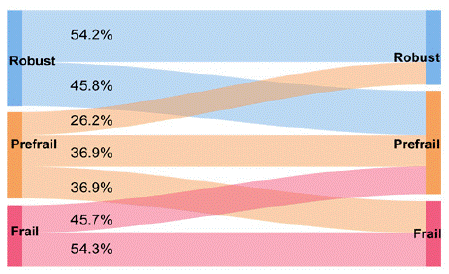
Figure 1: Changes in frailty states after 1-year follow-up among 183 older adults.
Factors Associated with Frailty Transitions
Among the 137 participants who were non-frail at baseline, 113(82.5%) participants remained stable (non-frail to non-frail) while24 (17.5%) participants worsened (non-frail to frail). Of the 46 older adults who were frail at baseline, 25 (54.3%) participants remained stable (frail to frail) and 21 (45.7%) participants improved (frail to non-frail). Among non-frail older adults, univariate analysis showed that there was a difference in age, walking speed, TUG, SPPB, serum albumin, serum hsCRP, cognition, and poly pharmacy between non-frail to non-frail older adults and non-frail to frail older adults (Table 2). However, only walking speed, TUG, SPPB, serum albumin, and serum hsCRP were independently related to worsening frailty in multivariate analysis adjusted for age and sex (Table 3). Among frail older adults, only cognition was related to frailty state improvement in both the univariate analysis (Table 2) and multivariate analysis adjusted for age and sex (Table 3).
Non-frail
Frail
Stability
Worsening
Stability
Improving
Age, M (IQR), year
82(80,86)
85(83,88)*
88(83.0,90.0)
85(82.5,88.5)
Sex, female, n (%)
65(57.5%)
13(54.2%)
15(71.4%)
15(60.0%)
BMI, x- (SD) , kg/m2
23.9(3.0)
25.3(3.4)
23.7(3.8)
23.5(3.6)
Grip strength, M (IQR), kg
22.1(18.6,25.6)
22.0(17.7,23.6)
18.1(14.4,22.1)
17.3(14.4,22.9)
Walking speed, M (IQR),m/s
0.91(0.71,1.07)
0.57(0.42,0.78)**
0.50(0.41,0.69)
0.62(0.48,0.81)
TUG,M (IQR),s
11.2(9.0,14.8)
15.8(13.1,25.7)**
22.7(16.3,30.0)
17.3(14.3,23.6)
SPPB,M (IQR), score
10(8,11)
5(4,9)**
4(3,6.5)
6(4,8)
MMSE=24, n(%)
11(9.7%)
6(26.1%)*
11(44.0%)
2(9.5%)*
MNA-SF <12 , n(%)
5(4.4%)
3(12.5%)
9(36.0%)
7(33.3%)
GDS-15 >5 , n(%)
8(7.1%)
4(16.7%)
10(47.6%)
7(28.0%)
Polypharmacy, n(%)
52(46.0%)
17(70.8%)*
16(76.2%)
21(84.0%)
CCI, M (IQR)
0(0,1)
1(0,2)
1(0.5,3)
2(1,2)
White blood cell count, M (IQR), 109/ L
5.4(4.4,6.6)
6.2(5.0,7.2)
5.7(4.5,6.3)
5.0(4.3,6.3)
hemoglobin, M (IQR), g/ L
137(128,147)
131(125,143)
128(117.3,142.8)
130(122,138)
Albumin, M (IQR), g/ L
42.9(41.1,44.9)
40.6(38.2,44.4)**
41.4(39.2,41.9)
40.0(38.9,42.6)
HsCRP, M (IQR),mg/L
1.34(0.79,2.49)
2.7(2.0,4.8)**
3.7(1.6,5.7)
1.5(0.6,4.1)
ESR, M (IQR),mm/h
13.3(10.0,21.8)
17.7(12.9,29.5)
29.2(17.0,36.7)
22.9(14.9,27.4)
*P<0.05;**P<0.001
Table 2: Comparison of baseline characteristics among different frailty transitions in non-frail and frail older adults.
Non-frail older adults
Walking speed
0.01(0.002,0.12)**
6.17(0.38,101.2)
TUG
1.08(1.02,1.15)*
0.97(0.92,1.03)
SPPB
0.79(0.68,0.92)*
1.07(0.85,1.34)
MMSE(0: <24;1:=24)
0.45(0.14,1.46)
6.73(1.15,39.19)*
Poly pharmacy
2.47(0.93,6.60)
0.57(0.12,2.62)
Albumin
0.78(0.64,0.94)*
0.98(0.79,1.23)
HsCRP
1.21(1.00,1.47)*
0.85(0.68,1.07)
*P<0.05;**P<0.001
Table 3: Risk factors associated with frailty transitions.
Predictive Value of Risk Factors Associated with Frailty Transitions
The ROC analysis showed that low walking speed, long TUG test time, low SPPB score, low serum albumin, and high serum hsCRP had predictive value for frailty state worsening, and good cognitive function had predictive value for frailty state improvement (Table 4).
AUC
95%CI
P value
Walking speed
0.81
0.73,0.89
0.000
TUG
0.77
0.67,0.87
0.000
SPPB
0.75
0.64,0.86
0.000
MMSE
0.69
0.53,0.85
0.034
Albumin
0.68
0.54,0.81
0.009
HsCRP
0.80
0.71,0.89
0.000
Table 4: Predictive values of the factors associated with frailty transitions.
Discussion
The influencing factors of frailty transition in the elderly remain unclear to date. In this study, the frailty state was dynamic during the follow-up period. Although most of the older adults remained in the same state or declined to a worsening frailty state, some had improved state. Walking speed, TUG, SPPB, serum albumin, and serum hsCRP were associated with worsening of the frailty state, whereas cognitive function was associated with its improvement. In addition, these factors were predictive of frailty state transitions.
Frailty was prevalent in 25.1% of our participants who had a mean age of 83.9 years (SD: 4.4), and this rate was comparable with the 23.3% prevalence rate in a previous study of participants with a mean age of 78.6 years (SD 7.1) that used the same frailty assessment tool [26]. Although it is common for frailty states of older adults to worsen or remain stable, our study showed that it is possible for individuals to change into lesser frailty states, consistent with previous findings [27-29]. This supports the notion that frailty is a dynamic and reversible state in older adults [11]. Thus, interventions for frailty state improvement in older adults with frailty should not be neglected.
Walking speed, TUG test, and SPPB are important and effective indicators of physical function in comprehensive geriatric assessment. Walking speed is a common item in frailty evaluation scales, especially in the frailty phenotype [2]. TUG is a widely used test that assessing the individual’s overall functional mobility [19]. Walking speed, balance, flexibility, and cognitive function are evaluated according to the SPPB [20]. A cross-sectional study showed that the time it took to complete TUG was longer in frail older adults than in pre-frail and non-frail older adults [30]. SPPB is a good predictor of mortality in older adults [31]. However, few studies included TUG and SPPB in exploring predictive factors of frailty transitions. This could be because the evaluation is complex and can thus be time consuming and costly in community screening, limiting its popularity. The current study observed that TUG and SPPPB were associated with frailty transition and had predictive value for a worsening frailty state. Considering their objectivity and comprehensiveness, SPPB and TUG may help identify older adults at high risk of worsening frailty and evaluate the effect of intervention on frailty.
Both cross-sectional and cohort studies have confirmed that inflammatory marker scan reflect frailty [32,33]. In the current study, serum hsCRPwas associated with frailty transition, and high hsCRP had a good predictive value for the worsening of the frailty state, consistent with previous studies [32,33]. Chronic inflammation may be an intermediate pathophysiological process that directly or indirectly leads to frailty [1]. Biological markers have more objective clinical value in predicting frailty. Frailty assessment tools, combined with biological markers, can better predict adverse outcomes [34,35]. HsCRP may be considered as a potential factor in developing a frailty assessment model in the future.
The current study found that the serum albumin was associated with frailty transition and predict the occurrence of worsening frailty. A decrease in serum albumin in a multicenter cohort of older men in the United States indicated a decreased likelihood of improvement in frailty states [36], and consistent findings were observed in the current study. Albumin is an important influencing factor of prognosis in older adults. In the model of 1 year post-discharge death in hospitalized older adults proposed by the University of California, San Francisco, a decline in serum albumin is an important predictor [37]. Albumin is partly indicative of nutritional status, and systematic reviews and meta-analyses have shown that malnutrition is associated with frailty among older adults [38], with malnutrition increasing the risk of frailty [39]. It can be seen that low serum albumin is not only associated with frailty, but also with poor prognosis [37]. Thus, more attention should be paid to elderly individuals with low serum albumin. One study suggested that protein supplementation improves muscle mass and physical function in frail and prefrail older adults with malnutrition [40]. From the perspective of community management of older adults, the serum albumin should be closely monitored, and early intervention should be provided to reduce adverse outcomes.
The current study showed that compared with older adults with decreased cognitive function, those with good baseline cognitive functions were more likely to improve from frailty to non-frailty. The association between cognitive function and frailty has been reported in previous studies [41-43]. A 10-year follow-up study of Mexican Americans found a significantly more impaired cognitive function in frail older adults than in non-frail older adults [44]. A possible explanation for the relationship between frailty and cognitive function is that the two have similar pathological mechanisms, such as chronic inflammation, nutritional imbalances, and cardiovascular diseases [45]. A previous study on the trajectory of decline in frailty and cognitive function showed that rapid growth of frailty coincides with a rapid decline in cognitive abilities, further supporting a common hypothesis of possible pathology [46]. In addition, another study of blood metabolite markers have found that there are overlapping markers for frailty and cognitive function. Older adults with good cognitive function may be more likely to make lifestyle changes, actively adjust their diet, and participate in exercise, all of which contribute to the improvement of their frailty state. Therefore, cognitive decline can be a good predictor of frailty transition, and improving cognitive function may improve the state of frailty.
Our study had some limitations. First, we used FRAIL, a selfassessment scale, to identify frailty, which may be affected by subjective factors of the participants. However, to date, there is no gold standard for the diagnosis of frailty, and there is no consensus on commonly used assessment tools. Second, 98.7% of participants’ education level was high school or above, although we considered that they well understood the content of the scale and provided objective and accurate results. This may limit the generalizability of our results to other older adults. Third, approximately 20% of the participants were lost to follow-up; however, there was no significant difference in participant characteristics between those who did and did not complete the follow-up. Lastly, this was a single-center study, which may explain why no association was observed between physical function and frailty transition in non-frail participants. A multicenter study with a large sample size is needed to evaluate the association between physical function and frailty transition.
Conclusions
Frailty is a dynamic state, and older adults can transition to less frail states. This observation cohort indicates that physical function, serum albumin, serum hsCRP, and cognition are predictive of frailty transitions. Importantly, the results indicate that frailty transition could be identified and controlled. Physical function, serum albumin, and serum hsCRP can be used to identify non-frail older adults at high risk of frailty progression and guide clinical decisions.
Acknowledgement
We would like to thank Editage (www.editage.cn) for English language editing.
Statement of Ethics
Written informed consent was obtained from all participants. This study was approved by the Research Ethics Committee of the Peking Union Medical College Hospital (PUMCH, JS2002). All methods were carried out in accordance with relevant guidelines and regulations.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Founding Sources
This work was supported by Irma and Paul Milstein Program for Senior Health, Milstein Medical Asian American Partnership Foundation. The funding body supported the data collection, analysis, and manuscript writing.
Authors Contributions
All authors contributed to the study conception and design. Material preparation and data collection was performed by Jiaojiao Li, Minglei Zhu, and Songqi Zhao. Material analysis was performed by Jiaojiao Li. The first draft of the manuscript was written by Jiaojiao Li. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data Availability Statement
Availability of data and materials: The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
- Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. The Lancet. 2013; 381: 752-762.
- Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology. Series A, Biological sciences and medical sciences. 2001; 56: M146-M157.
- Rockwood K. What would make a definition of frailty successful?. Age and ageing. 2005; 34: 432-434.
- Kojima G. Frailty as a Predictor of Future Falls Among Community-Dwelling Older People: A Systematic Review and Meta-Analysis. Journal of the American Medical Directors Association. 2015; 16: 1027-1033.
- Kojima G. Frailty as a predictor of disabilities among community-dwelling older people: a systematic review and meta-analysis. Disability and Rehabilitation. 2017; 39: 1897-1908.
- Crocker TF, Brown L, Clegg A, Farley K, Franklin M, Simpkins S, et al. Quality of life is substantially worse for community-dwelling older people living with frailty: systematic review and meta-analysis. Quality of Life Research. 2019; 28: 2041-2056.
- Shamliyan T, Talley KMC, Ramakrishnan R, Kane RL. Association of frailty with survival: A systematic literature review. Ageing Research Reviews. 2013; 12: 719-736.
- Collard RM, Boter H, Schoevers RA, Voshaar RCO. Prevalence of Frailty in Community-Dwelling Older Persons: A Systematic Review. Journal of the American Geriatrics Society. 2012; 60: 1487-1492.
- Ma L, Tang Z, Zhang L, Sun F, Li Y, Chan P. Prevalence of Frailty and Associated Factors in the Community-Dwelling Population of China. Journal of the American Geriatrics Society. 2017; 66: 559-564.
- Puts MTE, Toubasi S, Andrew MK, Ashe MC, Ploeg J, Atkinson E, et al. Interventions to prevent or reduce the level of frailty in community-dwelling older adults: a scoping review of the literature and international policies. Age and Ageing. 2017; 46: 383-392.
- Markle-Reid M, Browne G. Conceptualizations of frailty in relation to older adults. Journal of advanced nursing. 2003; 44: 58-68.
- Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, et al. Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. The journals of gerontology. Series A, Biological sciences and medical sciences. 2013; 68: 62-67.
- Wang M, Li T, Li C, Liu C, Lin W, Lin C, et al. Frailty, transition in frailty status and all-cause mortality in older adults of a Taichung community-based population. BMC Geriatrics. 2019; 19.
- Li C, Snih SA, Chou L, Karmarkar A, Kuo Y, Markides KS, et al. Frailty transitions predict healthcare use and Medicare payments in older Mexican Americans: a longitudinal cohort study. BMC Geriatrics. 2020; 20.
- Park CHK, Lee JW, Lee SY, Shim S, Kim SG, Lee J, et al. Characteristics of the “young-old” and “old-old” community-dwelling suicidal Ideators: A longitudinal 6-month follow-up study. Comprehensive psychiatry. 2019; 89: 67-77.
- Chuang T, Huang C, Lee C, Hsieh C, Hung Y, Hung C, et al. The differences of metabolic syndrome in elderly subgroups: A special focus on young-old, old-old and oldest old. Archives of gerontology and geriatrics. 2016;65:92-97. doi:10.1016/j.archger.2016.03.008
- KATZ S. Assessing Self-maintenance: Activities of Daily Living, Mobility, and Instrumental Activities of Daily Living. Journal of the American Geriatrics Society. 1983; 31: 721-727.
- Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019; 48: 601-601.
- Podsiadlo D, Richardson S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. Journal of the American Geriatrics Society. 1991; 39: 142-148.
- Treacy D, Hassett L. The Short Physical Performance Battery. Journal of physiotherapy. 2018; 64: 61.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975; 12: 189-198.
- Rubenstein LZ, Harker JO, Salvà A, Guigoz Y, Vellas B. Screening for undernutrition in geriatric practice: developing the short-form mini-nutritional assessment (MNA-SF). The journals of gerontology. Series A, Biological sciences and medical sciences. 2001; 56: M366-M372.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987; 40: 373-383.
- Kan GAV, Rolland YM, Morley JE, Vellas B. Frailty: toward a clinical definition. Journal of the American Medical Directors Association. 2008; 9: 71-72.
- Aprahamian I, Cezar NODC, Izbicki R, Lin SM, Paulo DLV, Fattori A, et al. Screening for Frailty With the FRAIL Scale: A Comparison With the Phenotype Criteria. Journal of the American Medical Directors Association. 2017; 18: 592-596.
- Lorenzo-López L, López-López R, Maseda A, Buján A, Rodríguez-Villamil JL, Millán-Calenti JC. Changes in frailty status in a community-dwelling cohort of older adults: The VERISAÚDE study. Maturitas. 2019; 119: 54-60.
- Setiati S, Laksmi PW, Aryana IS, Sunarti S, Widajanti N, Dwipa L, et al. Frailty state among Indonesian elderly: prevalence, associated factors, and frailty state transition. BMC Geriatrics. 2019; 19.
- Kojima G, Taniguchi Y, Iliffe S, Jivraj S, Walters K. Transitions between frailty states among community-dwelling older people: A systematic review and meta-analysis. Ageing Research Reviews. 2019; 50: 81-88.
- Ansai JH, Farche ACS, Rossi PG, Andrade LPD, Nakagawa TH, Takahashi ACDM. Performance of Different Timed Up and Go Subtasks in Frailty Syndrome. Journal of Geriatric Physical Therapy. 2017; 42: 287-293.
- Silva CDFR, Ohara DG, Matos AP, Pinto ACPN, Pegorari MS. Short Physical Performance Battery as a Measure of Physical Performance and Mortality Predictor in Older Adults: A Comprehensive Literature Review. International Journal of Environmental Research and Public Health. 2021; 18: 10612.
- Kane AE, Sinclair DA. Frailty biomarkers in humans and rodents: Current approaches and future advances. Mechanisms of Ageing and Development. 2019; 180: 117-128.
- Zhu Y, Liu Z, Wang Y, Wang Z, Shi J, Xie X, et al. C-reactive protein, frailty and overnight hospital admission in elderly individuals: A population-based study. Archives of gerontology and geriatrics. 2016; 64: 1-5.
- Sanchis J, Nunez E, Ruiz V, Bonanad C, Fernandez J, et al. Usefulness of Clinical Data and Biomarkers for the Identification of Frailty After Acute Coronary Syndromes. The Canadian journal of cardiology. 2015; 31: 1462- 1468.
- Howlett SE, Rockwood MR, Mitnitski A, Rockwood K. Standard laboratory tests to identify older adults at increased risk of death. BMC Medicine. 2014; 12.
- Pollack LR, Litwack-Harrison S, Cawthon PM, Ensrud K, Lane NE, Barrett- Connor E, et al. Patterns and Predictors of Frailty Transitions in Older Men: The Osteoporotic Fractures in Men Study. Journal of the American Geriatrics Society. 2017; 65: 2473-2479.
- Walter LC, Brand RJ, Counsell SR, Palmer RM, Landefeld CS, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. Jama. 2001; 285: 2987-2994.
- Verlaan S, Ligthart-Melis GC, Wijers SLJ, Cederholm T, Maier AB, Schueren MAEDVD. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults-A Systematic Review and Meta-Analysis. Journal of the American Medical Directors Association. 2017; 18: 374-382.
- Boulos C, Salameh P, Barberger-Gateau P. Malnutrition and frailty in community dwelling older adults living in a rural setting. Clinical nutrition. 2016; 35: 138-143.
- Park Y, Choi J, Hwang H. Protein supplementation improves muscle mass and physical performance in undernourished prefrail and frail elderly subjects: a randomized, double-blind, placebo-controlled trial. The American journal of clinical nutrition. 2018; 108: 1026-1033.
- Raji MA, Snih SA, Ostir GV, Markides KS, Ottenbacher KJ. Cognitive status and future risk of frailty in older Mexican Americans. The journals of gerontology. Series A, Biological sciences and medical sciences. 2010; 65A: 1228-1234.
- Kulmala J, Nykänen I, Mänty M, Hartikainen S. Association between Frailty and Dementia: A Population-Based Study. Gerontology. 2013; 60: 16-21.
- Magnuson A, Lei L, Gilmore N, Kleckner AS, Lin FV, Ferguson R, et al. Longitudinal Relationship Between Frailty and Cognition in Patients 50 Years and Older with Breast Cancer. Journal of the American Geriatrics Society. 2019; 67: 928-936.
- Samper-Ternent R, Snih SA, Raji MA, Markides KS, Ottenbacher KJ. Relationship Between Frailty and Cognitive Decline in Older Mexican Americans. Journal of the American Geriatrics Society. 2008; 56: 1845-1852.
- Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. The journals of gerontology. Series A, Biological sciences and medical sciences. 2014; 69: 1536-1544.
- Howrey BT, Snih SA, Middleton JA, Ottenbacher KJ. Trajectories of Frailty and Cognitive Decline Among Older Mexican Americans. The journals of gerontology. Series A, Biological sciences and medical sciences. 2020; 75: 1551-1557.
- Kameda M, Teruya T, Yanagida M, Kondoh H. Frailty markers comprise blood metabolites involved in antioxidation, cognition, and mobility. Proceedings of the National Academy of Sciences of the United States of America. 2020; 117: 9483-9489.