
Special Article: Sleep Apnea
Gerontol Geriatr Res. 2023; 9(1): 1083.
Air Sense Autoset for Her and Him
Kerl J*, Scheuermann J, Heyse D and Dellweg D
Fachkrankenhaus Kloster Grafschaft GmbH, Annostr. 1, 57392 Schmallenberg
*Corresponding author: Kerl JFachkrankenhaus Kloster Grafschaft GmbH, Schmallenberg, Schlaflabor, Sleep Laboratory, Germany
Received: January 09, 2023; Accepted: February 15, 2023; Published: February 22,2023
Abstract
Introduction: Several studies have shown that Obstructive Sleep Apnoea (OSA) results in gender specific or gender typical symptoms. The algorithms of automated CPAP devices (APAP) up to now focus on the therapy of typical male OSA symptoms. The “Air Sense 10 Autoset for Her” device was developed to provide a therapy with the focus on the treatment of typical female OSA symptoms. In this study the efficiency of the “Air Sense 10 Autoset for Her” Algorithm (AfH) in treatment of female as well as of male OSA symptoms was investigated in comparison to the standard APAP mode (ASstd).
Methods: In 40 women and 40 men a prospective randomized intraindividual cross-over trial was done where each OSA patient was half night treated with AfH algorithm and half night with ASstd algorithm. The flattening degree of each breath was calculated by deviding the relative inspiratory tidal volume by the relation of inspiration to expiration time (OC = Obstructive Coefficient© SOMNOmedics, Randersacker, Germany) in order to have analyzed all breaths of a whole night time recording because better treatment of inspiratory flattening is a major target of the AfH algorithm. The OC values under AfH/ASstd therapy were compared for each sleep stage separately for women and for men.
Results: In women the AfH algorithm provided better flattening treatment during N2 sleep (p<0.01, 104.115 breaths) and REM sleep (p<0.01, 32.348 breaths) while the ASstd algorithm was superior during N3 sleep (p<0.01, 57.286 breaths). No difference between the algorithms was observed during N1 sleep (15.803 breaths). In men the AfH algorithm was superior to the ASstd algorithm during each sleep stage (N1, N2, N3, REM, p<0.01, 211.440 breaths). When looking only at the number of breaths treated, independent from the sleep stage, in women AfH was superior to ASstd in 43.8% of the breaths, ASstd superior to AfH in 32.8% of the breaths and no algorithm preference was observed in 23.5% of the breaths. In men 52.7% of the breaths were better treated by AfH, 29.2% by ASstd, and in 18.1% no algorithm preference was observed.
Discussion: The AfH algorithm that has been especially developed for OSA treatment in women is only during N2- and REM-sleep more efficient in flattening treatment than the ASstd algorithm. The faster and more sensitive reaction to detected flow limitations did not provide better treatment in all sleep stages in women, but in the OSA treatment of men instead. The treatment of respiratory events was completely independent from this result and was similar effective in women as in men with no difference between the two APAP algorithms.
Introduction
In the sleep apnoea syndrome (OSA) most studies agree that there is a higher prevalence of OSA in men compared with women. The observed ratios vary in the range 2-4:1 (men/women). The under recognition of OSA in women may be explained by a different cluster of symptoms in women than in men. Another explanation is that women underreport the characteristic symptoms that are up to now associate with the syndrome like witnessed apneas, habitual snoring, and excessive daytime sleepiness.
Women suffering from OSA more often report symptoms like insomnia, restless legs, depression, nightmares, head eggs and muscle pain [1-3]. In addition women more often report limitation of the daytime performance and quality of life [4,5] and in comparison to men a more pronounced limitation at lower OSA severity degrees [6].
Several polysomnographic studies provided insight into the gender differences of OSA. The Apnoea-/Hypopnoea-Index (AHI) are lower in women compared to men [7-9]. Apnoea duration is shorter and the amount of hypopnoeas is higher in women than in men [10,11]. In women more flow limitated breaths were observed and the criteria of an Upper Airway Resistance Syndrome (UARS [14]) are more often fulfilled in women than in men [8,12,13]. Some studies report a lower AHI of women during non-REM sleep while no gender difference of the AHI during REM sleep was observed [7,9,11]. An influence of the body position on the appearance of respiratory events was only observed in men and not in women [7,15]. Sleep latency is longer in women than in men [7]. The amount on N3 sleep is higher while the number of arousals is lower in women compared to men [16].
On the basis of these gender differences ResMed Corporation (ResMed Ltd., Bella Vista, Sydney) has developed a female-specific ‘AutoSet for Her’ (AfH) algorithm. The AfH is designed to optimize the pressure response to the specific patterns of obstructive sleep disordered breathing seen in women.
The AfH algorithm is modified in comparison to the standard automated CPAP (APAP) algorithm, including an increased sensitivity to flow limitation, a slower, and lower, pressure rise and decay in response to flow limitation, a lower cap on the pressure response to obstructive apnoeas, and an adaptive minimum pressure [17]. Both algorithms are available in the same device.
In our study we also included male participants because we wanted to test whether the AfH algorithm may be also superior to the ASstd algorithm in the treatment of male OSA symptoms. The pathophysiological background for this interest is that flow limitation is reported to be the first symptom of a beginning OSA syndrome in men. When this early stage of OSA syndrome is ignored and not treated the symptoms become more severe with each year of missing treatment and obstructive hypopnoea and apnoea are added to the syndrome. Later on central apnoea may also occur. By splinting the upper airway collapsibility with an APAP therapy obstructive respiratory events are treated in the first line but maybe residual flow limitation will be untreated by ASstd or other standard APAP algorithms.
The aim of this study was to test the efficiency of the AfH algorithm in comparison to the standard APAP algorithm (ASstd) in women and in men by a prospective randomized intraindividual crossover trial.
Methods
The study protocol was approved by the ethics committee of the Philips University at Marburg (Germany) by registration number Studies 06/16. The study was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Written informed consent was obtained from all subjects. Registration as clinical trial was done under No. DRKS00012568 at the German Registry of clinical trials.
Recruitment of study participants was done among patients who came for the first time into our sleep laboratory for the purpose of validating a possible diagnosis of sleep disordered breathing. All patients underwent full night diagnostic polysomnography according to AASM standards to confirm the diagnosis as well as to avoid a first night effect during the polysomnography for study purposes in the second night.
The second AASM Polysomnography was done in the first therapy night of each patient. In the middle of the night the APAP mode was changed from AfH to ASstd or vice versa. In order to have no bias in the amounts especially of slow wave sleep and REM sleep in dependence on first/second half of the night in each group the test sequence AfHASstd was changed to ASstd AfH for half of the patients.
Polysomnography (PSG) was done by SOMNOscreen® PSG recorders together with the DOMINO® sleep laboratory software (SOMNOmedics, Randersacker, Germany). The below described algorithm for flow limitation analysis was programmed into the DOMINO® software.
Sleep stages were scored by an experienced board certified sleep technician. The flow signal from the AirSense 10 Autoset for Her was incorporated into the recorded data streams by the use of TxLink® devices (ResMed Ltd., Bella Vista, Sydney). The flow signal was analysed by a newly developed algorithm that calculates the obstructive coefficient© (SOMNOmedics, Randersacker, Germany, OC) for each breath by dividing the relative inspiratory tidal volume (relTVinsp, area under the positive slope of the flow signal) by the quotient of inspiration time to expiration time (tinsp/texp):
OC = relTVinsp / (tinsp/texp)
Division by (tinsp/texp) takes into account, that inspiration is compensatorily prolonged in the presence of flattening and airway obstruction [18,19]. The result of this calculation is a value of around 1000 when the area under the inspiratory flow curve is relatively large and no signs of flow limitation are seen in the contour of the inspiratory flow curve and a rounded sinusoidal shape is observed. In complete airway collapse the formula equals zero. All flow curve transitions between these two endpoints equal a value in the range 0-1000 as shown in (Figure 1).
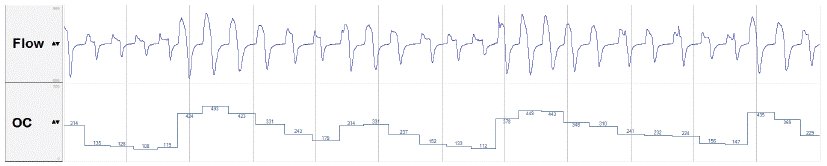
Figure 1:
The first analyses of the OC values have shown a strong gender dependent bias where the values of men were always larger than those of women. This is due to the fact that the relative inspiratory tidal volume is dependent from body size which is in most cases larger in men than in women. To compensate this difference without manipulating the measured effects the OC values were normalized to a relative mean value of all analyzed breaths during sleep (N1, N2, N3, REM) to 100%. By this transformation all outcome data (e.g. OC mean value comparison during N3 under AfH and ASstd therapy by t-Test reveals p=0.0089) are preserved and the same comparison with the percentage-transformed data reveals the same result (e.g. p=0.0089). This transformation of the raw OC data enabled us to compare outcome data from women to those of men. In general the OC values became comparable independent from body size.
Algorithm comparison of mean transformed OC values under AfH and ASstd therapy within the same sleep stage was done by t-Test. Mean transformed OC values under AfH and ASstd therapy of women and men within the same sleep stage were compared using analyses of variance (ANOVA).
Results
The study group is characterized in Table 1. An equal number of n=40 female and male participants was recruited. Female and male participants did not differ in age and Body Mass Index (BMI). The score in the Epworth Sleepiness Scale (ESS) was higher in women than in men (p=0.038) and Respiratory Disturbance Index (RDI) during diagnostic polysomnography was higher in men than in women (p=0.034).
Female
Male
p-value t-Test
Age
55.4 ± 13.1
50.8 ± 12.8
0.059
BMI
30.8 ± 6.3
30.3 ± 4.7
0.324
ESS Score
11.6 ± 4.3
9.7 ± 5.1
0.038
RDI (Diagn.)
16.6 ± 14.1
22.9 ± 16.0
0.034
Number
n=40
n=40
BMI Body Mass Index, ESS Score in the Epworth Sleepiness Scale, RDI (Diagn.) Respiratory Disturbance Index during diagnostic polysomnography
Table 1: Characterization of study group.
By changing the test sequence for half of the female and half of the male participants from AfH ASstd to ASstd AfH the amounts of all sleep stages were kept comparable in each subgroup of the study. No difference was observed in the half-night pooled data for each sleep stage for women and men (Table 2).
N1 AfH
N1 ASstd
p-value t-Test
Female (n=40)
7.8% (00:12:57h ± 00:10:09h)
8.2% (00:14:05h ± 00:10:44h)
0.315
Male (n=40)
9.4% (00:15:45h ± 00:19:04h)
8.9% (00:14:42h ± 00:17:09h)
0.398
N2 AfH
N2 ASstd
Female (n=40)
50.9% (01:24:07h ± 00:36:07h)
50.1% (01:25:47h ± 00:34:24h)
0.416
Male (n=40)
53.2% (01:29:12h ± 00:35:01h)
47.3% (01:17:55h ± 00:35:36h)
0.078
N3 AfH
N3 ASstd
Female (n=40)
27.3% (00:45:09h ± 00:23:11h)
26.5% (00:45:28h ± 00:29:58h)
0.479
Male (n=40)
22.7% (00:38:00h ± 00:25:58h)
26.0% (00:42:49h ± 00:26:55h)
0.209
REM AfH
REM ASstd
Female (n=40)
13.9% (00:22:53h ± 00:18:18h)
15.2% (00:26:00h ± 00:18:38h)
0.225
Male (n=40)
14.8% (00:24:47h ± 00:17:01h)
17.7% (00:29:10h ± 00:21:44h)
0.160
Table 2: Subgroup comparison of sleep stage amounts.
AfH and ASstd algorithms also were compared in their efficiency to treat breathing disorders like apnoea, hypopnoea and Respiratory Effort Related Arousals (RERA) which are usually summarized in the Respiratory Disturbance Index (RDI) of a polysomnographic report. In Table 3 the treatment efficiency of both algorithms is shown by comparing the RDI reduction. At first the diagnostic RDI of both groups (test sequence AfH ASstd vs. ASstd AfH) of women and men was tested for comparability by t-test. In women these values did not differ (RDI 15.7 vs. RDI 17.5, p=0.343) and even not in men (RDI 25.2 vs. RDI 20.6, p=0.213). After this test a RDI mean value for all women and all men was calculated. The RDI reduction in women and men by treatment with ASstd and AfH algorithm was either highly significant (p<0.01) in all groups.
Diagnostics
AfH treatment
ASstd treatment
p-value t-Test
women RDI 16.6 ± 14.1
RDI 5.4 ± 4.5
RDI 5.9 ± 4.6
0.309
men RDI 22.9 ± 16.0
RDI 7.3 ± 6.5
RDI 8.2 ± 7.3
0.281
Percentage of RDI reduction
women RDI 100%
60.2% ± 33.8%
59.3% ± 27.8%
0.445
men RDI 100%
58.8% ± 42.8%
51.0% ± 42.3%
0.206
RDI: Respiratory Disturbance Index, AfH: Autoset for Her Mode, ASstd: Standard Autoset Algorithm
Table 3: RDI reduction from diagnostic to treatment night.
In women as well as in men no difference is observed in the efficiency to reduce RDI between ASstd and AfH algorithm. With both algorithms a RDI reduction by 51.0 - 60.2% of the diagnostic value is reached.
All percentage transformed OC values were compared sleep stage by sleep stage between AfH and ASstd algorithm for women (Figure 2) and for men (Figure 3). OC values during wake were about 27% higher than during sleep in women and 26-29% higher in men. In women during N1 sleep no difference between the OC values under ASstd vs. AfH therapy was observed (n=15803 breaths). In the majority of N2 breaths (n=104115 breaths) the AfH therapy revealed higher OC values (p<0.01) than the ASstd therapy and provided a better treatment of flow limitations. During N3 sleep in women the ASstd algorithm was superior to the AfH algorithm (n=57286 breaths). During REM sleep our data show a better treatment of flow limitations by AfH algorithm compared to ASstd algorithm (n=32348 breaths).
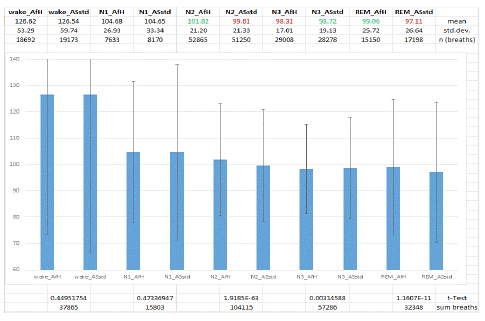
Figure 2: Percentage transformed OC values compared sleep stage by sleep stage between AfH and ASstd algorithm for women. AfH Autoset for Her Mode, ASstd standard Autoset algorithm, wake stage wake, N1 sleep stage non-REM 1, N2 sleep stage non-REM 2, N3 sleep stage non-REM 3, REM sleep stage REM, mean mean value, std.dev. standard deviation, n (breaths) number of breaths, sum breaths sum of breaths, green colour: significantly higher value in t-Test, red colour: significantly lower value in t-Test.
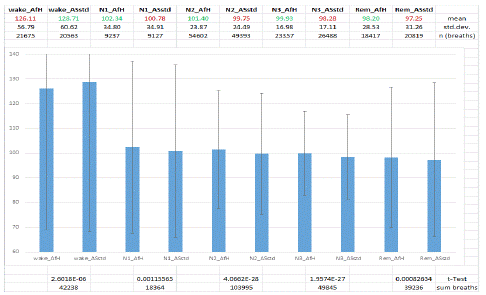
Figure 3: Percentage transformed OC values compared sleep stage by sleep stage between AfH and ASstd algorithm for men. AfH Autoset for Her Mode, ASstd standard Autoset algorithm, wake stage wake, N1 sleep stage non-REM 1, N2 sleep stage non-REM 2, N3 sleep stage non-REM 3, REM sleep stage REM, mean mean value, std.dev. Standard deviation, n (breaths) number of breaths, sum breaths sum of breaths, green colour: significantly higher value in t-Test, red colour: significantly lower value in t-Test.
In men the AfH algorithm was superior to the ASstd algorithm in the treatment of flow limitation in each of the four sleep stages N1 (n=18364 breaths), N2 (n=103995 breaths), N3 (n=49845 breaths), and REM (n=39236 breaths). In each sleep stage OC values under AfH therapy were significantly higher than under ASstd therapy (p<0.01).
Comparing the results for women with those of the men (Table 4) the maximum airway patency that is reflected by the highest OC values is observed in women during N1 sleep, independent from the APAP algorithm used for treatment. The lowest OC values are observed in REM sleep with the ASstd algorithm both in women and in men. Analysis of Variance (ANOVA) showed no difference between these two lowest values. During REM sleep the AfH algorithm is superior to the ASstd algorithm, independent from gender. This is also true for N2 sleep that makes up most of the total sleep time (46.7 - 51.7%). Looking at the columns of Table 4 in the sleep stage sequence from N1 to REM sleep in men there is always the same OC distribution observed from the maximum value during N1 sleep stepwise down to the minimum value during REM sleep with both APAP algorithms. The same is observed in women with ASstd treatment but not with AfH treatment. With AfH treatment the lowest OC value in women is observed during N3 sleep instead of REM sleep.
sleep stage
F_AfH
F_ASstd
M_AfH
M_ASstd
N1
*104.68 ± 26.93
(n=7633, 7.3%)*104.65 ± 33.34
(n=8170, 7.8%)102.34 ± 34.80
(n=9237, 8.7%)100.78 ± 34.91
(n=9127, 8.6%)N2
101.82 ± 21.20
(n=52865, 50.5%)*99.61 ± 21.33
(n=51250, 48.9%)101.40 ± 23.87
(n=54602, 51.7%)*99.75 ± 24.49
(n=49393, 46.7%)N3
*98.31 ± 17.01
(n=29008, 27.7%)98.72 ± 19.13
(n=28278, 27.0%)99.93 ± 16.98
(n=23357, 22.1%)*98.28 ± 17.11
(n=26488, 25.0%)REM
99.06 ± 25.72
(n=15150, 14.5%)*97.11 ± 26.64
(n=17198, 16.4%)98.20 ± 28.53
(n=18417, 17.4%)*97.25 ± 31.26
(n=20819, 19.7%)F_AfH female participants treated with Autoset for Her Mode, F_ASstd female Participants treated with standard Autoset algorithm, M_AfH male participants treated with Autoset for Her Mode, M_ASstd male Participants treated with standard Autoset algorithm, N1 sleep stage non-REM 1, N2 sleep stage non-REM 2, N3 sleep stage non-REM 3, REM sleep stage REM, *Analysis of Variance (ANOVA) revealed no difference between these two values of a row while all other values are significantly different to each other.
Table 4: Mean percentage transformed OC values sorted by gender, APAP algorithm, and sleep stage.
Discussion
The aim of this study was to test the AfH algorithm in comparison to the ASstd algorithm in their efficiency to treat flow limitation (inspiratory flattening) as well as respiratory events like apnoea, hypopnoea and RERA by a prospective randomized crossover trial in women as well as in men suffering from OSA. The study design was chosen in order to overcome the limitations of the already existing AfH study from McArdle et al. 2015 [17] that could not exclude a night-to-night variation and was carried out with a very poor measurement method for inspiratory flattening. In the McArdle study flow limitation was assessed by using the sponsor’s (ResMed Ltd.) flow limitation tool to automatically identify whether a breath is flow limited or not. So in the McArdle study the natural transition between a rounded flow contour without any signs of flow limitation to a serious flattened contour with only a minimum tidal volume left is ignored and instead in the sponsor’s (ResMed Ltd.) flow limitation tool an unknown threshold is defined to decide whether an inspiratory flow shape was flattened or not.
In our trial we provided maximum transparency by using an algorithm for flow contour analysis with a free accessible mathematical formula that can be used by anyone and that will always reveal the same results when applied to the same dataset [19]. By applying this algorithm to each breath of the study any difference in treatment of inspiratory flattening could be detected. By the choice of this tool we assured that we were able to evaluate this most important feature of the AfH algorithm in comparison to the ASstd algorithm by including any transitional stages of flow limitation in each breath of the study.
To avoid a night-to-night variation that may have influence on the results we have done a crossover split night trial and assured by changing the test sequence from AfH ASstd to ASstd AfH for half of the participants to have comparable amounts of N1-, N2-, N3-, and REM-sleep in each subgroup of the study.
Our results show that both algorithms are effective in the treatment of respiratory events like aponeas, hypopnoeas, and RERAs without any difference in women as well as in men. But the most important feature of the AfH algorithm is its design to be more sensitive to flow limitations. Instead of requiring three consecutive flow-limited breaths, as in the ASstd algorithm, it reacts upon the first identified flow-limited breath with a moderate pressure raise. This feature was refined with regard to the increasing evidence of a higher prevalence of flow limitation in women compared to men.
In the treatment of flow limitation our study revealed heterogeneous results in women. The AfH algorithm provided better flow limitation therapy during N2- and REM sleep which together make up the majority of all breaths during sleep. But during N3 sleep the ASstd algorithm was superior to the AfH algorithm while no difference was observed during N1 sleep. The good REM sleep response of the AfH algorithm may be an effect of the moving minimum APAP pressure to which pressure can drop during sleep periods without respiratory events. This leads to minimized phases of inappropriate pressure drops during REM sleep that might occur in the ASstd algorithm. In sleep stage N2 the highest airflow resistance is observed in OSA patients [19]. Under these conditions the AfH algorithm seems to provide a better treatment quality of flow limitations compared to the ASstd algorithm in women. The flow limitations during N3- and N1-sleep result from lower airflow resistance values and are better treated by the ASstd algorithm in N3 sleep or comparable good by both algorithms in N1 sleep.
In men the AfH algorithm provided better flow limitation treatment than the ASstd algorithm in any sleep stage. This result supports the hypothesis that after successful treatment of obstructive respiratory events like apnoea and hypopnoea also men are in need to have a sensitive algorithm for fast reaction to detected residual flow limitations. It appears that the OSA syndrome in women bears a strong resemblance to early-stage OSA syndrome in men that is usually ignored by the male patients and remains untreated for years. When men start to suffer from OSA the syndrome already has developed to a more severe degree of sleep disordered breathing so that the treatment acts upon a far more advanced level of airway obstruction with apnoea and hypopnoea as predominant symptoms. Up to now the male-biased treatment strategies target these respiratory events. But when the occurrence of apnoea and hypopnoea is successfully prevented in men our results indicate that there is still residual flow limitation to treat what is done better by the AfH algorithm than by the ASstd algorithm.
Although most CPAP titration clinical protocols recommend the elimination of apneas, hypopneas and snoring [20], there is no agreement on the need to correct residual flow limitation. There are very few data that suggest that flow limitation should be eliminated during CPAP titration [21]. With the use of the AfH algorithm, a method is now available that covers the entire range of requirements for the elimination of nocturnal breathing disorders by APAP treatment.
References
- Kapsimalis F, Kryger M. Sleep breathing disorders in the U.S. female population. Journal of women's health. 2009; 18: 1211–1219.
- Baldwin CM, Kapur VK, Holberg CJ, Rosen C, Nieto FJ. Associations between gender and measures of daytime somnolence in the Sleep Heart Health Study. Sleep. 2004; 27: 305–311.
- Valipour A, Lothaller H, Rauscher H, Zwick H, Burghuber OC, et al. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep. A clinical population study using the sleep disorders questionnaire. Sleep. 2007; 30: 312–319.
- Sampaio R, Pereira MG, Winck JC. Psychological morbidity, illness representations, and quality of life in female and male patients with obstructive sleep apnea syndrome. Psychology, health & medicine. 2012; 17: 136–149.
- Ye L, Pien GW, Ratcliffe SJ, Weaver TE. Gender differences in obstructive sleep apnea and treatment response to continuous positive airway pressure. Journal of clinical sleep medicine. 2009; 5: 512–518.
- Young T, Hutton R, Finn L, Badr S, Palta M. The gender bias in sleep apnea diagnosis. Are women missed because they have different symptoms?. Archives of internal medicine. 1996; 156: 2445–2451.
- Basoglu OK, Tasbakan MS. Gender differences in clinical and polysomnographic features of obstructive sleep apnea. A clinical study of 2827 patients. Sleep Breath. 2017; 22: 241-249.
- Mohsenin V. Gender differences in the expression of sleep-disordered breathing. Role of upper airway dimensions. Chest. 2001; 120: 1442–1447.
- O'Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. American journal of respiratory and critical care medicine. 2000; 161: 1465–1472.
- Leech JA, Önal E, Dulberg C, Lopata MA. A Comparison of Men and Women with Occlusive Sleep Apnea Syndrome. Chest. 1988; 94: 983–988.
- Ware JC, McBrayer RH, Scott JA. Influence of sex and age on duration and frequency of sleep apnea events. Sleep. 2000; 23: 165–170.
- Polo-Kantola P. Breathing during sleep in menopause a randomized, controlled, crossover trial with estrogen therapy. Obstetrics & Gynecology. 2003; 102: 68–75.
- Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A Cause of Excessive Daytime Sleepiness. In: Chest. 1993; 104: 781–787.
- Pépin JL, Guillot M, Tamisier R, Lévy P. The Upper Airway Resistance Syndrome. In: Respiration; international review of thoracic diseases. 2012; 83: 559–566.
- Mohsenin V. Effects of gender on upper airway collapsibility and severity of obstructive sleep apnea. Sleep medicine. 2004; 4: 523–529.
- Valencia-Flores M, Bliwise DL, Guilleminault C, Rhoads N, Clerk A. Gender differences in sleep architecture in sleep apnoea syndrome. Journal of sleep research. 1992; 1: 51–53.
- McArdle N, King S, Shepherd K, Baker V, Ramanan D, et al. Study of a Novel APAP Algorithm for the Treatment of Obstructive Sleep Apnea in Women. Sleep. 2015; 38: 1775–1781.
- Schwartz AR, Schneider H, Smith PL, McGinley BM, Patil SP, et al. Physiologic phenotypes of sleep apnea pathogenesis. Am J Respir Crit Care Med. 2011; 184: 1105-1106.
- Kerl J, Noeke P, Heyse D, Dellweg D: Normal and obstructive breathing physiology during sleep. Sleep and Breathing. 2021; 25: 1335-1341.
- Rapoport DM. Methods to stabilize the upper airway using positive pressure. Sleep. 1996; 19: S123–S13.8.
- Meurice JC, Paquereau J, Denjean A, Patte F, Series F. Influence of correction of flow limitation on continuous positive airway pressure efficiency in sleep apnoea/hypop-noea syndrome. Eur Respir J. 1998; 11: 1121–7.