Special Article: Sleep Apnea
Gerontol Geriatr Res. 2023; 9(1): 1086.
Relationship between AHI, Oximetric Parameters and Comorbidities in Adult and Elderly Patients with Moderate-to- Severe OSAS: An Observational Study
Lo Iacono CAM*; Ippolito M; Achilli A; Losacco R; Ianni T; Martino F; Di Diego I; De Angelis C; Gobbi F; Ettorre E
Department of ENT and Audiology, University of Ferrara, Italy
*Corresponding author: Lo Iacono CAMDepartment ENT and Audiology, University of Ferrara, Via Aldo Moro, 8, 44124 Ferrara, Italy.
Received: February 17, 2023 Accepted: March 24, 2023 Published: March 31, 2023
Abstract
Background: OSAS is a chronic disease characterised by recurrent episodes of apnoea/hypopnoea, which generate chronic intermittent hypoxia, the main etiopathogenetic factor of OSAS-related comorbidities: cerebro-cardiovascular, metabolic and cognitive deficits. Although it represents a major public health problem, it is under-diagnosed. At polygraphy, the Apnoea-Hypopnoea Index (AHI) identifies the number of obstructive episodes, while the oximetry parameters ODI, T90 and minimum and mean SpO2 measure the severity of chronic intermittent hypoxia.
Aim of the Study: To test: 1 the usefulness of oximetry parameters in identifying adult and elderly patients with moderate-severe OSAS at higher risk of comorbidities; 2 whether there is a statistically significant association between oximetry parameters and CIRS-G in the elderly; 3 whether CPAP use >4 hours is a protective factor for comorbidities in the elderly.
Materials and Methods: A total of 220 patients with moderate-severe OSAS without respiratory comorbidities were enrolled and data on: anthropometric variables, lifestyle, comorbidities and drug therapy were collected. The MMSE and sleep questionnaires (ESS, PSQI, AIS) were administered. The AHI and oximetric parameters were extracted from the polygraph. A value of <30 and ≥30 was chosen as cut-off for ODI, for T90 <20% and ≥20%, for minimum SpO2 <75% and ≥75% and for mean SpO2 <91% and ≥91%. Pcs were divided into two groups of age <65 and ≥65; for the group with age ≥65, the Comorbidity Index (CI) and Severity Index (IS) of the CIRS-G Scale were calculated. In addition, the patients, who accepted CPAP, were followed up at 1-3-6 months, at 1 year and once a year.
Results: A high (OR >1) and statistically significant (p<0.05) risk emerged of having diabetes mellitus with ODI ≥30; heart disease with mean SpO2 <91%; hypertension, diabetes mellitus and heart disease with T90 ≥20%. The increased risk for cognitive deficits and heart disease with AHI ≥30 was also statistically significant (p<0.05). Linear regression analysis showed that the T90 >20% -SpO2 minimum <75% interaction is positively associated with CI of the CIRS-G scale (p<0.05) and that CPAP use >4 hours is negatively associated with CI and IS of the CIRS-G scale (p<0.05).
Conclusions: In moderate-severe OSAS, oximetry parameters, beyond the AHI, are useful in identifying those adult and elderly patients at higher risk of comorbidities. During the obstructive event, having a minimum SpO2 <75% for a long time (T90 ≥20%) increases CI in the elderly. Increased compliance with CPAP, the gold standard treatment of OSAS, is a protective factor for comorbidities in the elderly.
Introduction
Obstructive Sleep Apnoea Syndrome (OSAS) is a disease with a high predominance in the general population, with a strong impact on health and quality of life and with significant social and health implications. It is often underdiagnosed in the geriatric patient, since the specific symptoms of OSAS such as snoring, daytime sleepiness, concentration deficit, memory impairment, asthenia, nocturia, irritability, mood alterations are considered as multifactorial effects due to the frequent comorbidities and the polypharmacotherapy. OSAS is characterized by repeated episodes of complete (apnoea) or partial (hypopnea) upper airway obstruction, causing increased intrathoracic negative pressure, sleep fragmentation, chronic intermittent hypoxia, sympathetic hypertonicity, endothelial dysfunction, and systemic inflammation. These mechanisms facilitate the occurrence of metabolic and cerebro-cardiovascular diseases, typical comorbidities of patients with moderate- severe OSAS. Since OSAS worsens the vulnerability of the geriatric patient by reducing its survival duration, it is essential to diagnose and treat it. CPAP (Continuous Positive Airway Pressure) therapy improves not only nocturnal hypoxemia values, an etiopathogenetic factor of associated comorbidities, but also the clinical symptoms related to OSAS.
Abbreviations: AHI: Apnea-Hypopnea Index; AIS: Athens Insomnia Scale; BMI: Body Mass Index; CI: Comorbidity Index; CIRS-G: Cumulative Illness Rating Scale for Geriatrics; CPAP: Continuous Positive Airway Pressure; CRP: C-Reactive Protein; DBMA: Disease Burden Morbidity Assessment; EDS: Excessive Daytime Sleepiness; ESS: Epworth Sleepness Scale; MMSE: Mini Mental State Examination; msOSAS: OSAS Moderate-to-Severe; NCM: Nocturnal Cardiorespiratory Monitoring; ODI: Oxigen Desaturation Index; OSA: Obstructive Sleep Apnea; OSAS: Obstructive Sleep Apnea Syndrome; PSQI: Pittsburgh Sleep Quality index; PHQ-9: Patient Health Questionnaire-9; RDI: Respiratory Disturbance Index; SI: Severity Index; SpO2: Oxygen Saturation; Minimum SpO2: Minimal Oxygen Saturation; Medium SpO2: Average Oxygen Saturation; T90: Recording Time of NCM with Oxygen Saturation <90%; V-EGF: Vascular-Endothelial Growth Factor
Foreword and Purpose of the Study
ISTAT [1] [Italian national Institute of Statistics–TN] expects that in 2045 over a third of the Italian population will be aged 65 or over and that the aging process will be associated with a drastic accumulation of chronic diseases and syndromes. The result will be a progressive increase in the predominance of comorbidities. This, although frequently associated with disability and vulnerability, typical conditions of advanced age, should be considered differently. Recent guidelines [2] on multimorbidity and comorbidity in the elderly patient have not only highlighted specific chronic diseases, which reduce quality of life and increase mortality, such as the Obstructive Sleep Apnoea Syndrome, but have also validated the use in clinical practice of Cumulative Illness Rating Scale for Geriatrics (CIRS-G), a tool which measures the type of simultaneous pathologies, the level of severity and the functional disability they cause.
OSAS is a chronic disorder attributable to alterations of ventilatory mechanics and respiration, characterized by recurrent episodes of apnoea/hypopnea, which generate intermittent chronic hypoxia. The latter is the main factor contributing to the pathogenesis of OSAS-related comorbidities, i.e. cerebro-cardiovascular, metabolic and neuro-cognitive pathologies. Given the high mortality rate, preventing these induced pathologies using the tools at our disposal appears to be crucial.
The AHI index, although it allows to identify the number of obstructive episodes that occur in an hour of sleep by classifying OSAS into mild, moderate and severe, does not, however, detect the severity of the single obstructive event as it does not evaluate duration or degree of desaturation. The extent of nocturnal hypoxemia can be measured with other oximetric parameters recorded on the polygraph: ODI (Oxygen Desaturation Index), T90 and minimum and medium SpO2. The ODI expresses the number of episodes of desaturation ≥3% per recording hour; the T90 the percentage of the recording time spent with O2 saturation <90%; the minimum SpO2 the lowest O2 saturation levels achieved during the recording; the medium SpO2 the medium O2 saturation achieved during the recording. A multicentre study [3], carried out in Spain, has demonstrated that C-PAP, after a use of at least 4 hours per night for 3 months improves daytime sleepiness and reduces a cerebro- cardiovascular risk, metabolic disorders and cognitive deficits in both adult and elderly patients. Poor patient compliance may limit the benefits. However, the treatment of choice for moderate to severe obstructive sleep apnoea is C-PAP.
Therefore, the purpose of the study is to verify:
- What is the relationship between AHI, oximetric parameters and comorbidities in adult and elderly patients with moderate-severe OSAS and identify those patients at a higher risk of comorbidity;
- Whether there is a statistically significant association between the oximetric parameters considered and CIRS-G in the elderly;
- If CPAP usage time >4 hours is a protective factor for comorbidity in the elderly population.
Population, Materials and Methods
We have carried out the study at the Sleep Disorders Clinic of the UOC [Complex Operating Unit – TN] of Geriatrics of the "Umberto I" Polyclinic in Rome directed by Prof. E. Ettorre and examined 275 patients, registered from January 2015 to July 2021; of these we excluded: 8 patients with mild OSAS and/or positional OSAS, 25 patients due to the presence of respiratory comorbidities (chronic obstructive pulmonary disease or asthma) and 22 patients due to incomplete data. By means of the prior informed consent procedure, we have enrolled 220 patients diagnosed with moderate-severe OSAS, eligible for treatment with CPAP. Of these, 101 patients accepted CPAP and only 90 followed up. We have divided the sample into two age groups: <65 years (adults) and ≥65 years (elderly) and collected their complete medical history including current drug therapy and administered questionnaires to investigate daytime sleepiness and sleep quality (Epworth Sleepiness Scale; Pittsburgh Sleep Quality Index; Athens Insomnia Scale). We have extracted data relating to age, sex, quality of life (smoking and alcoholic habits), anthropometric variables (height, weight, BMI, neck circumference, waist circumference) and recorded the associated comorbidities: arterial hypertension, type 2 diabetes mellitus, dyslipidaemia, myocardial infarction, arrhythmias, heart failure, dysthyroidism, neurological pathologies and cognitive deficits with the MMSE (Mini-Mental Test Examination).
For patients aged ≥65 we have calculated the CIRS-G scale (Cumulative Illness Rating Scale for Geriatrics) [4]. It evaluates the clinical and functional severity of 14 pathologies on an anatomical basis (organ by organ). Each item is considered according to levels of increasing severity from 0 (absent pathology) to 4 (very severe pathology). From it we obtain two measures:
• Severity Index (SI), which results from the average of the scores of the first 13 categories. The maximum possible score is 5.
• Comorbidity index (Comorbidity Index - CI), which represents the number of categories with a score equal to or greater than 3 (referring only to the first 13 categories). The maximum score obtainable is 13. Item 14 (psychiatric-behavioural) is excluded from the count in order to avoid misunderstandings between mental health and cognitive ability.
We have had all patients undergo full nocturnal cardiorespiratory monitoring using Embletta MPR and Nox T3 equipment. These devices detect peripheral oxygen saturation (SpO2), nasal airflow, thoracoabdominal movements, heart rate, snoring, and body position. We have therefore considered the recordings as valid when performed on a total sleep time of at least 4 hours and interpreted by integrating the automatic analysis of the RemLogic or Noxturnal software with the manual analysis of the medical staff. Apnoea was defined as absence or reduction in nasal airflow ≥90% for a period of at least 10 seconds, and hypopnea as a reduction in nasal airflow ≥30% for a period of at least 10 seconds associated with an oxyhemoglobin desaturation ≥3%. From these respiratory events we have obtained the AHI (number of apnoeas and hypopneas per hour of sleep), the ODI (number of episodes of oxyhemoglobin desaturation ≥3% of baseline per hour of sleep), and the oximetry parameters: the T90, the minimum and average SpO2 and the average of the desaturation peaks. Then we chose the oximetric parameter cut-offs, already validated in the literature [5], i.e. <e ≥30 for ODI, <e ≥20% for T90, <e ≥75% for minimum SpO2, < and ≥91% for average SpO2. Finally, the patients who accepted the CPAP treatment were followed up at 1-3-6 months, 1 year and once a year.
Statistical Analysis
All statistical analyses have been carried out using Stata 17 software (IBM, Armonk, New York). Continuous variables have been expressed on average ± Standard Deviation. Categorical variables have been expressed in absolute frequencies and compared percentages using Pearson's X² test.
A logistic regression model was used to test the association between AHI, oximetric parameters (independent variables) and comorbidities (dependent variables) by calculating the Odds Ratio (ORs) and the corresponding 95% confidence interval (95% CI). The ORs is the ratio between the frequencies of occurrence of the "illness" event in exposed and unexposed subjects; when OR is greater than 1 the factor under examination is implicated in the onset of the disease.
The degree of association between T90-minimum SpO2, independent variables, and CIRS-G, dependent variable, has been analysed with a linear regression model, calculating the coefficient β. The sign of the β coefficient indicates the direction of the relationship: the positive sign determines the agreement between the variables.
Similarly, the degree of association between the use of CPAP, the independent variable, and CIRS- G, the dependent variable, has been analysed with a linear regression model, calculating the coefficient β. The sign of the β coefficient indicates the direction of the relationship: the negative sign determines the disagreement between the variables.
A value of p<0.05 has been accepted as statistically significant.
Results
Of the 220 patients, 122(55%) are adults (age <65 years) and 98(45%) are elderly (age ≥65years); 72(33%) are females and 148(67%) males. 97(44%) had moderate OSAS (AHI =15 =30), while 123(56%) had severe OSAS (AHI ≥30); 63(29%) had daytime sleepiness (Epworth test >10); 147(73%) snore. The average weight of the sample is 94.12kg (±25.50); the average BMI is 32.55(±8.31)kg/m2; the average neck circumference is 42.55(±8.99)cm; the average waist circumference is 113.65(±17.17)cm. All the clinical characteristics and comorbidities of the sample under examination, distinguished by age ≥65 and <65, are summarized in (Tables 1 and 2).
Total sample N=220
Age =65 anni N=98
Age <65 anni N=122
P-value
n° (%)
n° (%)
n°(%)
-
Sex
-Male
148 (67)
61 (41)
87 (59)
-Female
72 (33)
37 (51)
35 (49)
0.154
Middle Age
61,18 (±12.24)
71,95 (±5,65)
52,52 (±8,72)
-
Average weight
94,12 (±25,50)
83,19 (±17,59)
102,89 (±27,48)
0.000
kg
kg
kg
Average BMI
32,55 (±8,31)
29,71 (±5,61)
34,83 (±9,39)
0.000
kg/m2
kg/m2
kg/m2
Average neck circumference
42,95 (±8,99)
41,43 (±3,88)
44,20 (±11,49)
0.048
cm
cm
cm
Average waist circumference Lifestyle
113,65 (±17,17)
108,78 (±13,07)
117,60 (±19,06)
0.001
cm
cm
cm
-smoke
51 (23)
20 (39)
31 (61)
0.076
-alcohol
142 (64)
57 (40)
85 (60)
0.621
Snoring
147 (73)
65 (56)
82 (44)
0.155
Daytime sleepiness (test Epworth>10)
63 (29)
30 (48)
33 (52)
0.338
Table 1: Anthropometric characteristics and lifestyle of the sample under examination.
Total sample N=220
Age =65 anni N=98
Age <65 anni N=122
P-value
n° (%)
n° (%)
n° (%)
-
Hypertension
144 (65)
68 (69)
76 (62)
0.272
Acute myocardial infarction
21 (10)
10 (10)
11 (9)
0.766
Heart pathologies (arrhythmias, heart failure)
31 (14)
23 (23)
8 (7)
0.000
Cognitive Impairment
21 (10)
21 (21)
0
0.000
Diabetes Mellitus
46 (21)
23 (23)
23 (19)
0.403
Neurological Pathologies
33 (15)
19 (19)
14 (11)
0.102
Dyslipidemia
81 (37)
36 (37)
45 (37)
0.982
Dysthyroidism
35 (16)
17 (17)
18 (15)
0.601
Table 2: Comorbidities in the sample under examination.
The variables analysed for which a statistically significant P value is found are average weight, average BMI, average neck and waist circumference, heart diseases and cognitive deficits (p<0.005).
Table 3 shows the OSAS parameters detected at the polygraph divided by age ≥65 and <65. Of 220 patients enrolled, 90(41%) accepted nocturnal ventilation with C-PAP, while 130(59%) refused. The use of C-PAP is higher in patients over 65 years of age (n. 54 of which 25 for more than a year). Furthermore, this difference in the sample under examination is statistically significant (p<0.001) Table 3.
Total sample N=220
Age =65 anni N=98
Age <65 anni N=122
P-value
n° (%)
n° (%)
n° (%)
AHI
>15<30 (moderate) (severe)
97 (44)
38 (39)
59 (48)
0.155
=30
123 (56)
60 (61)
63 (52)
ODI
<30
97 (44)
41 (42)
56 (46)
0.546
=30
123 (56)
57 (58)
66 (54)
Minimum SpO2
<75%
80 (36)
32 (33)
48 (39)
0.305
=75%
140 (64)
66 (67)
74 (61)
Average SpO2
<91%
75 (34)
31 (32)
44 (36)
0.491
=91%
145 (66)
67 (68)
78 (64)
T90
<20%
141 (64)
68 (69)
73 (60)
0.142
=20%
79 (36)
30 (31)
49 (40)
Use of C-PAP
<1 year
57 (26)
29 (51)
28 (49)
0.000
>1 year
33 (15)
25 (76)
8 (24)
No C-PAP
130 (59)
44 (34)
86 (66)
Table 3: AHI, Oximeter Parameters, C-PAP in the Sample under Examination.
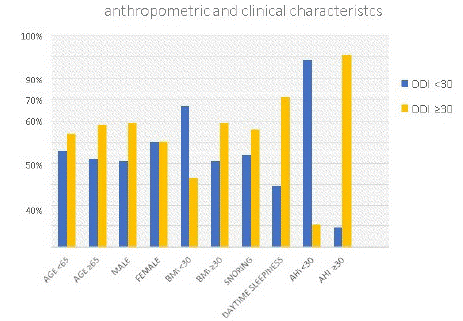
The anthropometric and clinical characteristics of the sample for AHI have been weighed with values between 15 and 30 and ≥30. The group with AHI ≥30 had age ≥65, mainly males, BMI ≥30, more snoring and sleepiness (Graph 1).
graph 1: AHI
Furthermore, the socio-demographic and clinical characteristics of the sample have been evaluated for the various oximetric parameters, comparing for the ODI patients with values <30 and ≥30; for T90 patients with values <20% and ≥20%, for minimum SpO2 patients with values <75% and ≥75%; for medium SpO2 patients with values &≥91% and ≥91%.
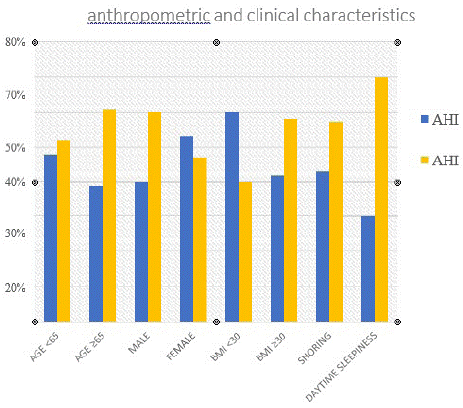
Of the 220 patients, 97(44%) reported an ODI <30 and 123(56%) an ODI ≥30. The ODI ≥30 group had age ≥65, male predominance, BMI ≥30, excessive sleepiness and snoring, and AHI ≥30 (Graph 2). Of the 220 patients, 80(36%) had a minimum SpO2 <75% and 140(64%) a minimum SpO2 ≥75%.

graph 2: ODI
The group with minimum SpO2 <75% has age <65, equal number of males and females, BMI ≥30, less snoring and daytime sleepiness, and AHI ≥30 (Graph 3).
graph 3: Minimum SpO2.
Of the 220 patients, 145(66%) had a medium SpO2 ≥91% and 75(34%) had a medium SpO2 <91%. The group with medium SpO2 <91% has age <65, female predominance, BMI ≥30, less snoring and daytime sleepiness, and AHI values ≥30 (graph 4).
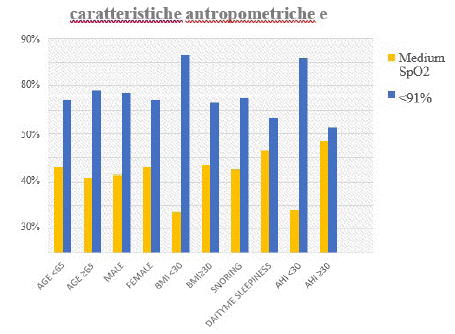
graph 4: Medium SpO2.
Of the 220 patients, 141(64%) had a T90 <20% and 79(36%) a T90 ≥20%. The group with T90 ≥20% had age <65, female predominance, BMI ≥30, less snoring and daytime sleepiness, and AHI ≥30 (Graph 5).
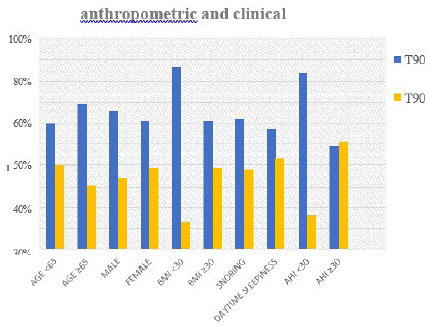
Figure 5: T90.
The comparison between AHI and oximetric parameters shows that patients with the same AHI have variable oximetric values. Considering AHI >15 <30, ODI and T90 agree (approximately 80% have ODI <30 and T90 <20%), while minimum and medium SpO2 do not agree (approximately 80% have minimum SpO2 ≥75% and medium SpO2 ≥91%). Instead, if we consider AHI ≥30, only ODI agrees (90% have ODI ≥30), for the other oximetric parameters there is no difference: about 50% have minimum and medium SpO2 values and T90 values above the threshold and 50% have values below the threshold (graphs 2-3-4-5). The comparison is statistically significant p<0.001 (Table 4).
AHI >15<30
AHI =30
P-value
n°
%
n°
%
-
ODI <30
ODI =3086
1189
1111
1129
910.000
Minimum SpO2 <75% Minimum SpO2 =75%
17
8018
8263
6051
490.000
Medium SpO2 <91%
Medium SpO2 =91%17
8018
8258
6547
530.000
T90 <20%
T90 =20%81
1684
1660
6349
51
0.000
Table 4: AHI and oxitric parameters in the sample under examination.
The comparison between T90 and the remaining oximetric parameters shows that T90 ≥20% agrees with ODI ≥30, minimum SpO2 <75% and medium SpO2 <91% (p-value<0.001) (Graph 6).
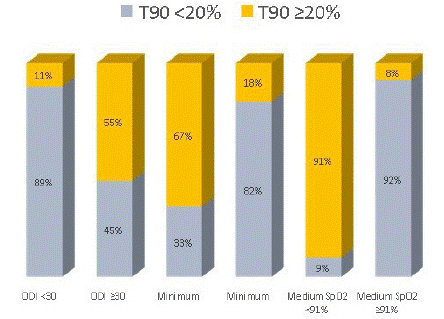
graph 6: T90 e oximetric parameters.
Similarly, minimum SpO2 <75% also agrees with ODI ≥30, T90 ≥20%, and medium SpO2 <91%. (p- value <0.001) (graph 7).
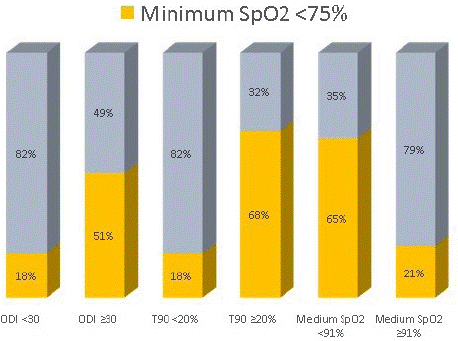
graph 7: Minimum SpO2 e oximetric parameters.
Graphs 8 and 9 show the different percentage of comorbidities between the cut-offs chosen for the oximetric parameters. Minimum SpO2 and its cut-offs are equally distributed among the comorbidities, while medium SpO2, T90 and ODI and the related cut-offs are more differentiating: 70% of those with dyslipidemia have a T90 <20%, 77% of those with dyslipidemia has a medium SpO2 ≥91%, 70% of those with heart disease and cognitive impairment have ODI ≥30.
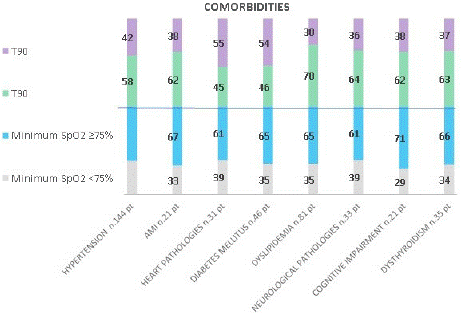
graph 8: Comorbidities and oximetry parameters.
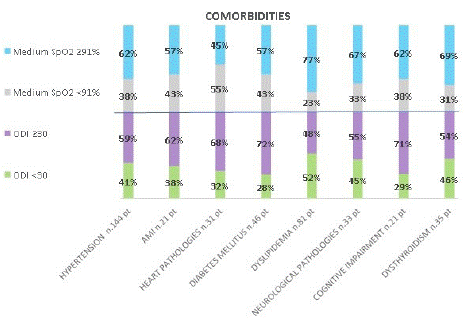
graph 9: Comorbidities and oximetry parameters.
In the total sample, a logistic regression model has been used to identify the association between AHI, oximetric parameters and comorbidities, which estimated the odds ratios of having arterial hypertension, myocardial infarction, arrhythmias, heart failure, dyslipidemia, stroke, TIA, cognitive impairment and dysthyroidism (dependent variables) with abnormal oximetric values or AHI (independent variables).
A high risk (greater than 1) has emerged for almost all oximetric parameters in determining the comorbidities under examination. Specifically, the ORs is statistically significant (p<0.05) for ODI ≥30 and diabetes mellitus; for average SpO2 <91% and heart diseases; for T90 ≥20% and arterial hypertension, diabetes mellitus, heart diseases. No associations greater than 1, statistically significant, have been found between minimum SpO2 and the pathologies considered. The association between the AHI index and cognitive deficits and cardiac pathologies is statistically significant (p<0.05). Furthermore, patients with medium Spo2 <91% and AHI ≥30 had a lower risk of having dyslipidemia (p<0.05). All results are reported in (Table 5 and 6).
Variables
Odds ratio
(95% CI)
P-value
HYPERTENSION
OD I =30
1.44
(0.83-2.52)
0.200
SpO2 min <75%
1.26
(0.44-1.42)
0.438
SpO2 medium <91%
1.73
(0.31-1.06)
0.079
T90 =20%
2.14
(1.16-3.97)
0.015
AHI =30
1.22
(0.70-2.14)
0.477
AMI
ODI =30
1.31
(0.52-3.31)
0.562
SpO2 min <75%
0.86
(0.45-3.00)
0.762
SpO2 medium <91%
1.51
(0.27-1.65)
0.375
T90 =20%
1.11
(0.44-2.80)
0.826
AHI =30
1.31
(0.52-3.31)
0.562
HEART PATHOLOGIES (arrhythmias, heart failure)
ODI =30
1.79
(0.80-4.00)
0.156
SpO2 min <75%
1.12
(0.41-1.94)
0.770
SpO2 medium <91%
2.74
(0.17-0.79)
0.010
T90 =20%
2.49
(1.15-5.37)
0.020
AHI =30
3.12
(1.28-7.58)
0.012
DYSLIPIDEMIA
ODI =30
0.61
(0.35-1.06)
0.078
SpO2 min <75%
0.88
(0.64-2.00)
0.673
SpO2 medium <91%
0.45
(1.19-4.07)
0.012
T90 =20%
0.59
(0.33-1.06)
0.077
AHI =30
0.56
(0.32-0.98)
0.041
Table 5: Laboratory values at hospital admission.
Variables
Odds ratio
(95% CI)
P-value
DIABETES MELLITUS
ODI =30
2.36
(1.17-4.80)
0.017
SpO2 min <75%
0.92
(0.55-2.15)
0.802
SpO2 medium <91%
1.66
(0.31-1.17)
0.133
T90 =20%
2.65
(1.36-5.13)
0.004
AHI =30
1.29
(0.66-2.51)
0.447
NEUROLOGICAL PATHOLOGIES
ODI =30
0.94
(0.45-1.97)
0.864
SpO2 min <75%
1.16
(0.40-1.84)
0.695
SpO2 medium <91%
0.96
(0.47-2.28)
0.921
T90 =20%
1.02
(0.47-2.21)
0.953
AHI =30
1.25
(0.59-2.67)
0.556
COGNITIVE IMPAIRMENT
ODI =30
2.1
(0.78-5.65)
0.139
SpO2 min <75%
0.68
(0.55-3.98)
0.437
SpO2 medium <91%
1.21
(0.33-2.09)
0.684
T90 =20%
1.11
(0.43-2.80)
0.826
AHI =30
8.68
(1.97-38.25)
0.004
DYSTHYROIDISM
ODI =30
0.34
(0.45-1.91)
0.833
SpO2 min <75%
0.9
(0.52-2.38)
0.781
SpO2 medium <91%
0.87
(0.53-2.50)
0.717
T90 =20%
1.07
(0.50-2.25)
0.868
AHI =30
0.92
(0.45-1.91)
0.833
Table 6: Logistic regression model: Odds ratio between comorbidities (dependent variables) and oximetry parameters, AHI (independent variables).
Stratifying the sample by age, greater or less than 65, in the group with age ≥65 (n. 98 patients) the linear regression model has been used to estimate the association between the interaction of the minimum T90-SpO2 oximetric parameters and the CIRS-G_IC. The model was adjusted for daytime sleepiness, assessed with the Epworth test, and snoring, two typical symptoms of OSAS, but also frequent in old age due to polypharmacotherapy and pharyngeal muscle laxity.
The result is a positive and statistically significant (p<0.05) association between T90 ≥20% - minimum SpO2 <75% and ≥75% (independent variables) and comorbidities quantified with the CIRS- G_IC scale (dependent variable). In our sample the variability range of the CIRS-G_IC scale goes from 0 to 3 and the comorbidity index increases on average by 0.86 with T90 ≥20% - minimum SpO2 <75%, “worst” cut-offs of the oximetric parameters, but it increases on average by 0.77 even with only T90 ≥20% (Table 7).
Variables
β*
(95% CI)
P-value
CIRS-G_IC
-T90 <20% e SpO2 min =75%
0.52
(-0.54-1.10)
0.075
-T90 =20% e SpO2 min <75%
0.86
(0.19-1.53)
0.012
-T90 =20% e SpO2 min =75%
0.77
(0.25-1.50)
0.043
*β: coefficient of variation
Table 7: Linear regression model adjusted for daytime sleepiness (Epworth test >10) and snoring: CIRS-G_IC (Comorbidity Index), dependent variable, and minimum T90-SpO2, independent variable.
In the same linear regression model adjusted for daytime sleepiness and snoring, the Severity Index (SI) of the CIRS-G scale (dependent variable) was also analysed in order to estimate its association with the interaction of the oximetric parameters T90-minimum SpO2 (independent variable). The results are not statistically significant; bordering on significance is the association between T90 ≥20%-minimum SpO2 <75% and CIRS-G_IS (p-value = 0.057). In our sample, the variability range of the CIRS-G_IS scale goes from 0 to 1 and the severity index increases on average by 0.18 with "worst" cut-offs of oximetric parameters (Table 8).
Variables
β*
(95% CI)
P-value
CIRS-G_IS
-T90 <20% e SpO2 min >75%
0.07
(-0.09-0.23)
0.383
-T90 =20% e SpO2 min <75%
0.18
(-0.01-0.36)
0.057
-T90 =20% e SpO2 min =75%
0.12
(-0.08-0.32)
0.229
*β: coefficient of variation
Table 8: Linear regression model adjusted for daytime sleepiness (Epworth test >10) and snoring: CIRS-G_IS (Severity Index), dependent variable, and minimum T90-SpO2, independent variables.
Considering that in the sample examined, patients aged ≥65 use CPAP more (54 patients for more than 4 hours a night) with the linear regression model, the degree of association between CPAP compliance and Comorbidity Index (CI) and the Severity Index (IS) of the CIRS-G scale. Assuming that poor sleep quality is a factor able to establish a CPAP adherence, the model was adjusted for the Athens Insomnia Scale, a self-assessment test of sleep quality. The use of CPAP is the independent variable, CIRS_IC (Comorbidity Index) and CIRS_IS (Severity Index) the dependent variables. The results are shown in (Table 9). The association is negative and adherence to CPAP results in an average reduction of 0.19 in the comorbidity index (p-value<0.05) and an average reduction of 0.64 in the severity index (p-value<0.05) (in the sample under examination the variability range of the CIRS-G_IC scale is 0-3; the variability range of the CIRS-G_IS scale is 0-1).
Variables
β*
(95% CI)
P-value
CIRS-G_IC -use of CPAP >1 year
-0.19
(-0.31 -0.07)
0.002
CIRS-G_IS -use of CPAP >1 year
-0.64
(-1.06 -0.22)
0.003
*β: coefficient of variation
Table 9: Linear regression model adjusted for Athens Insomnia Scale: CIRS-G_IC (Comorbidity Index), CIRS-G_IS (Severity Index) dependent variables, and CPAP, independent variable.
Discussion
Moderate-to-severe OSAS (msOSAS) is associated with an increased risk of comorbidities: cerebro- cardiovascular diseases, metabolic disorders, and cognitive deficits. It therefore represents a significant public health problem due to the health, social and economic impact linked to late diagnoses, the increase in morbidity and mortality and the treatment of comorbidities itself.
Current guidelines recommend the use of the AHI index in polygraphy to diagnose and classify the degree of severity of OSAS. As reported in the literature, some patients with similar msOSAS and AHI may have a different risk of comorbidity. This is thought to be due to a different degree of chronic nocturnal hypoxia, i.e. an arterial oxygen saturation <75%(SpO2) and/or total sleep time spent with an O2 saturation <90%(T90) for a period equal to or greater than 20% of the sleep time. A previous study [6] found that patients with T90 >20% and minimum SpO2 <75% had increased concentrations of C-Reactive Protein (CRP), higher platelet number and increased endothelial stiffness, decisive inflammatory etiopathogenetic factors for the onset of OSAS-related comorbidities.
In our study, by observing the variability of oximetric parameters in patients with the same AHI (Table 4), we have verified the utility of ODI, minimum and medium SpO2, T90 with the relative cut- offs in predicting the risk of comorbidities. With the logistic regression model (Tables 5 & 6) we have demonstrated that the risk of comorbidity is high and is statistically significant (p<0.05) for arterial hypertension, diabetes mellitus, heart diseases with T90 values ≥20%, medium SpO2 <91% and ODI ≥30. Also in case neurological pathologies, myocardial infarction, cognitive deficits there is a greater risk, although statistically insignificant. AHI was instead associated with cardiac pathologies and cognitive deficits (p<0.05). In contrast to the literature [7], in our sample dysthyroidism is not associated with oximetric parameters, probably due to the smallness of the sample. Paradoxically, the study showed that patients with medium SpO2 <91% and AHI =30 have a lower risk of having dyslipidemia (p<0.05) and consequently atherosclerosis. This observation, as indicated by Lavie et al., [8] is due to chronic intermittent hypoxia, which generates a protective mechanism through the release of V-EGF (Vascular-Endothelial Growth Factor) and the development of collateral vessels, reducing the complications deriving from atherosclerosis.
Although in the study, among all the oximetric indexes, the most suitable was found to be the T90, the need to consider the oximetric parameters as a whole in the polygraphy, beyond the AHI, to identify the subgroup of msOSAS patients, adult and elderly, with higher risk of comorbidities and worse prognosis, results as evident.
Several studies have shown that despite the growing interest in OSA-related comorbidities, their association is underdiagnosed in the middle-aged and elderly population. It is also known that the clinical manifestations and consequences of OSAS vary with age and gender and that the Apnoea-Hypopnea Index (AHI) increases with age [9-12], making it insensitive. Therefore, in clinical practice in the absence of a pathological AHI cut-off in the elderly population, the global evaluation of oximetric parameters appears to be more important. In our study we have supposed that in the elderly population a low saturation (minimum SpO2 <75%) for a long time (T90 ≥20%) is the cause of a higher number of comorbidities. Then, using the CIRS-G scale we have quantified comorbidities across the CI and IS. Then, using the linear regression model, adjusted for daytime sleepiness and snoring, we have found that the two oximetric parameters and in particular the T90 are associated with the score on the CIRS-G scale reflecting a dose response effect between the oximetric parameters and the number and severity of comorbidities (Table 7-8): the number of comorbidities increases by 28% with T90 ≥20% - SpO2 <75% (p-value<0.05), while the severity index increases by 18% (p-value=0.057). It is deduced that, although the clinical and symptomatic variability of the elderly may hide OSAS, OSAS-related chronic intermittent hypoxia worsens the prognosis of patients with comorbidities [13], also having an impact on disability. Furthermore, the result strengthens the hypothesis that oximetric parameters are useful in differentiating msOSAS phenotypes at higher risk of concomitant chronic diseases. There are few studies regarding OSA-related comorbidities in the geriatric population. Hallè et al. [14] use a self-report questionnaire, the DBMA (Disease Burden Morbidity Assessment), to quantify the comorbidity, our study instead associates the diagnosis of msOSAS with an objective scale of comorbidity validated in the clinical setting, the CIRS-G, whose data are collected by medical personnel.
The comorbidity and severity index deriving from the CIRS-G scale do not evaluate cognitive deficits, but the correlation between minimum SpO2 and MMSE scores in the elderly population is widely demonstrated in the literature [15,16]; as a matter of fact, as O2 saturation decreases, the MMSE scores decrease, proving that the obstructive event also worsens cognitive functions.
The treatment of choice for msOSAS is CPAP. Despite the progressive improvement of CPAP devices, adherence to its use is an ongoing challenge for sleep medicine specialists. If, on the one hand, there are several studies on the adult population, which demonstrate the benefits of CPAP treatment in reducing long-term complications, on the other hand, few studies have been carried out in the elderly population [17]. In our sample, 90 patients accepted nocturnal ventilation and of these only 15% used CPAP for more than a year. Furthermore, as reported in a previous study [18], we have observed that the greatest CPAP compliance is in the age group =65. Therefore, in order to verify the benefits of CPAP in this age group, we used the linear regression model assuming that the greater CPAP compliance has an effect on the comorbidity index and on the severity index deriving from the CIRS-G scale. The model has been adjusted for the Athens Insomnia Scale, a self-report test of sleep quality. The result shows that, with the same quality of sleep, the use of CPAP is a protective factor for comorbidities, also decreasing the degree of severity: p-value<0.05 (Table 9).
Already in 2015 a cohort study [19] reported that msOSAS not treated with CPAP was associated with a high risk of mortality in the elderly population and that adequate treatment with CPAP could reduce this risk. In elderly people, diagnosing and treating OSAS is essential, since the resulting comorbidities negatively impact the quality of life, increasing medical consultations, length of hospital stay and mortality rate.
The results of the study have an interesting clinical implication, but it must be specified that the analysis was carried out on a small number of patients; therefore, it is necessary to carry out further studies on a greater number of patients to confirm the data.
Conclusions
By analysing the data emerging from our study, the oximetric indexes, reflecting the state of nocturnal hypoxemia, are, in association with the AHI, useful to identify adult and elderly patients at higher risk of comorbidities. In fact, a better phenotypic characterization of patients with msOSAS would allow individualizing the therapy and reducing long-term complications. CPAP is the gold standard of treatment for obstructive sleep apnoea and its use, as demonstrated, has a real benefit even in the elderly population. Therefore, the future challenge is to implement adherence to this device.
References
- Istituto Nazionale di Statistica. Il futuro demografico del Paese.
- Linee Giuda-314-SIGG_Multomorbidità e Polifarmacoterapia_rev (2021).
- Martínez-García MÁ, Amilibia J, Chiner E, Queipo C, De Atauri MJD, et al. Sleep apnea in elderly individuals. Assistance activity (2002–2008) in Spain. Arch Bronconeumol. 2010; 46: 502–507.
- Fabio Salvi, Mark D Miller, Adele L Towers, Valeria Morichi, e Paolo Dessi Fulgheri, Linee guida per la valutazione della scala Modified Cumulative Illness Rating Scale. J Am Geriatr Soc. 2008; 56; Appendice S1.
- Gonzalo Labarca, Jorge Dreyse, Constanza Salas, Francisca Letelier, Alexia Schmidt, et al. Clinical utility of oximetric parameters to identify a high-risk phenotype of moderate-severe obstructive sleep apnea (osa). The Clinical Respiratory Journal. 2020; 14: 1166-1175.
- Yilmaz Avci A, Avci S, Lakadamyali H, Can U Hypoxia and inflammation indicate significant differences in the severity of obstructive sleep apnea within similar apnea-hypopnea index groups. Sleep Breath. 2017; 21: 703–71.
- Gamaldo AA, Beydoun MA, Beydoun HA, Liang H, Salas RE, et al. Sleep disturbances among older adults in the United States, 2002-2012: nationwide inpatient rates, predictors, and outcomes. Front Aging Neurosci. 2016; 8: 266.
- Lavie L, Lavie P. Ischemic preconditioning as a possible explanation for the age decline relative mortality in sleep apnea. Med Hypotheses. 2006; 66: 1069–1073.
- Launois SH, Pépin JL, Levy PA. Sleep apnea in the elderly: A specific entity?. Sleep Med Rev. 2007; 11: 87–97.
- Lévy P, Pépin JL, Malauzat D, Emeriau JP, Léger JM. Is Sleep Apnea Syndrome in the Elderly a Specific Entity?. Sleep. 1996; 19: S29–S38.
- Collop NA. The significance of sleep-disordered breathing and obstructive sleep apnea in the elderly. Chest. 1997; 112: 867–868.
- Norman D, Loredo JS. Obstructive Sleep Apnea in Older Adults. Clin Geriatr Med. 2008; 24: 151-165.
- Marrone O, Lo Bue A, Salvaggio A, Dardanoni G, Insalaco G, et al. Comorbidities and survival in obstructive sleep apnoea beyond the age of 50. Eur J Clin Invest. 2013; 43: 27e33.
- Robichaud-Halle L, Beaudry M, Fortin M. Obstructive sleep apnea and multi- morbidity. BMC Pulm Med. 2012; 12: 60.
- Terri Blackwell, Kristine Yaffe, Alison Laffan, Susan Redline, Sonia Ancoli-Israel, et al. Associations between sleep- disordered breathing, nocturnal hypoxemia, and subsequent cognitive decline in older community-dwelling men: the Osteoporotic Fractures in Men Sleep Study. Multicenter study. J Am Geriatr Soc. 2015; 63: 453-61.
- Adam P Spira, Terri Blackwell, Katie L Stone, Susan Redline, Jane A Cauley, et al. Sleep-disordered breathing and cognition in older women. Multicenter Study. J Am Geriatr Soc. 2008; 56: 45-50.
- Tomas Posadas, Grace Oscullo, Enrique Zaldívar, Alberto Garcia-Ortega, José Daniel Gómez-Olivas, et al. Treatment with CPAP in Elderly Patients with Obstructive Sleep Apnoea. Journal of Clinical Medicine. 2020; 17: 546.
- James M Parish, Philip J Lyng, Joyce Wisbey. Compliance with CPAP in erderly patients with OSAS. Sleep Medicine. 2000; 1: 209-214.
- Qiong Ou, Yong-Chi Chen, Sheng-Qing Zhuo, Xiang-Ting Tian, Chun-Huan He, et al. Continuous Positive Airway Pressure Treatment Reduces Mortality in Elderly Patients with Moderate to Severe Obstructive Severe Sleep Apnea: A Cohort Study. Plos One. 2015; 10: e0127775.