
Research Article
Gerontol Geriatr Res. 2023; 9(2): 1088.
Guillain Barré Syndrome in the Elderly: Does Age Affect the Course?
Kirchner RJ¹*; Alessandro L¹; Castiglione JI¹; Varela F¹; Barroso F¹
¹Department of Neurology, Neurological Research Institute Dr. Raúl Carrea, Fundación para la Lucha contra las Enfermedades Neurológicas de la Infancia (FLENI), Argentina
*Corresponding author: Kirchner RJ Department of Neurology, Neurological Research Institute, Montañeses 2325, Buenos Aires, C1428, Argentina. Email: rjkirchner@fleni.org.ar
Received: March 27, 2023 Accepted: May 06, 2023 Published: May 13, 2023
Abstract
Objectives: Describe the characteristics of Guillain Barré Syndrome in patients older than 60 years (Latin American population).
Methods: Retrospective analysis of 141 patients with diagnosis of Guillain Barré Syndrome. 43 in the elderly group (>60 years) and 98 patients in the young group (18–59 years). Clinical characteristics, electrodiagnosis, cerebrospinal fluid, treatment and prognosis (12 months of follow-up) were compared between groups.
Results: A longer delay from the prodrome to the disease (median in days 14 vs 7; p=0.04). greater involvement in deep sensitivity (72.5% vs 29.6%; p=0.001) and ataxia (30.2% vs 13.2%; p=0.01) in the elderly.
In the follow-up, the prognosis was similar using the Hughes scale (12 months: ederly group 0.22 vs young group 0.29; p=0.6).
Conclusions: A longer delay from the prodromal event to the onset of Guillain Barré Syndrome in the elderly could be interpreted as a more insidious presentation in the context of immunosenescence.
A greater compromise of deep sensitivity and ataxia must be taken into consideration for an adequate approach to rehabilitation.
With our results we cannot conclude that age is an independent risk factor for worse prognosis.
Keywords: Guillain barré syndrome; Elderly; Immunosenescence
Abbreviations: GBS: Guillain Barré Syndrome; MRC: Medical Research Council Sum Score Scale; CSF: Lumbar Cerebrospinal Fluid; EMG: Electromyogram; ICU: Intensive Care Unit
Introduction and objectives
Guillain Barré syndrome (GBS) is an acute-onset immune-mediated polyradiculoneuropathy. Worldwide, it is the most common cause of acute neuromuscular weakness in all age groups (from 4 months to 95 years), with an overall incidence of 0.5-2 per 100,000 people [1]. Although some studies report an increase in incidence with age [2], other authors describe a bimodal distribution with a peak in the fourth and sixth decade of life [3,4] with a decrease after 80 years of age [5-7].
Establishing an individual prognosis is difficult due to the variability of the disease, which depends on multiple factors, for example: electrophysiological variants [8] timing of treatment initiation [9] and others inherent to the patient such as age. In relation to this last point, studies have been carried out to assess specific age groups, mainly in the pediatric population [10], although analyzes aimed at GBS in the elderly are scarce [11-13]. In these studies, some characteristics of this age range were described, such as a higher incidence of axonal variants and a worse prognosis in terms of disability and mortality.
Since the World Health Organization defines the elderly as any person over 60 years of age, the objective of this study is to describe the clinical, electrophysiological and prognostic characteristics of GBS in Latin American population.
Materials and Methods
A retrospective analysis of medical records of patients older than 18 years with diagnosis of GBS, treated at a referral center (Buenos Aires, Argentina), in the period from June-2006 to June-2021.
Patients were evaluated by neurologists from our neuromuscular disorders clinic or hospital neurologist, and the diagnosis of GBS was established according to the criteria developed at the National Institute of Neurological Disorders and Stroke (NINDS) [14].
Demographic characteristics such as age, sex, and diabetes (comorbidity for neuropathy) were evaluated.
Within the clinical characteristics, the presence of prodromes (infectious conditions or vaccinations), the delay from the prodrome to the onset of the symptoms, degree of motor deficit at the nadir using the Medical Research Council (MRC) sum score scale [15], loss of tendon reflexes, cranial nerve involvement, alteration of the sensory system (neuropathic pain, paresthesia and hypoesthesia for protopathic touch, bathyesthesia and palesthesia), ataxia and dysautonomia (alterations in heart rate, blood pressure, bladder and gastrointestinal) were assessed.
Patients were evaluated by analysis of lumbar Cerebrospinal Fluid (CSF) and Electromyogram (EMG) with conduction velocities of at least 3 motor nerves and 3 sensory nerves [16]. To categorize these results and establish the electrophysiological subtype, Hadden et. al diagnostic criteria [17] were used.
The treatment performed in the different groups was analyzed: gamma globulin, plasmapheresis, or wait and see; Also, if the patients spent part of the hospitalization in the Intensive Care Unit (ICU) for the reason they required it: ventilatory failure or dysautonomia with hemodynamic instability.
To assess the prognosis, the condition was classified according to the Hughes disability scale [18] at the time of hospitalization as: mild (score 0 to 2) or severe (score 3 to 5). After hospital discharge, a follow-up of at least 1 year was carried out, evaluating the patients with the same scale at 6 and 12 months. Futhermore, the total days of hospitalization, days of hospitalization in the ICU, use of ventilatory assistance if required, and mortality were detailed.
The data of the variables corresponding to the group of patients older than 60 years (elderly group) were compared with the group of patients aged 18-59 years (young group). Differences of the variables between both groups were compared using student t test, Fisher test, and Pearson chi-squared test, accordingly. Software Rstudio was used to perform statistical analysis.
Results
Demographic and Clinical Variable Results [Table N°1]
Variables
Young group 18 - 59 years (n=98)
Elderly group = 60 years (n=43)
P value
Demographic
Median Age (range)
38.5 (18 - 59)
67 (60 - 86)
Male gander; n (%)
58 (59,1%)
22 (51.2%)
Clinical
Diabetes; n (%)
5 (5.1%)
6 (13.9%)
0.07
Prodromal event, n (%)
71 (72,4%)
26 (60.4%)
0.12
- Respiratory infection; n (%)
29 (40,8%)
17 (65.3%)
0.2
- Gastroenteritis; n (%)
29 (40,8%)
7 (26.9%)
0.09
Delay prodom - GBS onset (median in days)
7.5
14,00
0.04
Pain; n (%)
61 (62,2%)
27 (62.8%)
0.9
Sensory Deficit; n (%)
81 (82.5%)
40 (93%)
0.08
- Protopatic touch; n (%)
35 (43.2%)
20 (50%)
0.2
- Bathyesthesia / Palesthesia; n (%)
24 (29,6%)
29 (72.5%)
0.001
Paresthesia; n (%)
25 (25.1%)
15 (34.9%)
0.5
Ataxia; n (%)
13 (13,2%)
13 (30.2%)
0.01
Preserved tendon reflexes; n (%)
11 (11.2%)
1 (2,3%)
0.08
Cranial nerves; n (%)
49 (50%)
17 (39.5%)
0.2
VII cranial nerve; n (%)
46 (46%)
13 (30%)
0.06
Lower cranial nerves (IX, X, XI, XII); n (%)
9 (9.1%)
3 (7%)
0.6
Dysautonomias; n (%)
31 (31,6%)
12 (27.9%)
0.5
Nadir MRC score Avarege (Median)
47,1 (52)
47.8 (50)
0.5
Nadir Hugues score Avarege (Median)
2,5 (2)
2,8 (3)
0.2
- Mild GBS; n (%)
58 (59,1%)
19 (44,2%)
0.09
- Severe GBS; n (%)
40 (40,8%)
24 (55,8%)
0.09
GBS: Guillain Barre Síndrome; MRC: Medical Research Council sum score scale
Table 1: Demographic and clinical variables.
We included 141 patients with GBS. 43 patients were included in the elderly group (51.2% male) and 98 patients in the young group (59.1% male). The elderly group presented a median age of 67 years. The distribution of the patients in subgroups was as follows: 60 - 69 years: n=29; 70 to 79 years: n=10; older than 80 years: n=4.
A tendency to higher incidence of prior diabetes was evidenced in the elderly group (13.9% vs 5.1%; p value= 0.07).
Regarding clinical variables, similar presentation of prodromal events were observed between groups (elderly group: 60.4% vs young group: 72.4%; p=0.12), although with longer delay from the prodrome to the onset of the disease in the elderly (median in days 14 vs 7; p=0.04) [see Graph N°1].
According to motor deficit, at the time of admission the mean MRC sum score (15) was 47.8 (range 28 - 60), similar to the young group of 47.1 (range 6 - 60). However, the Hughes Disability Scale [17] showed a trend of severe GBS (score 3 to 5) in the elderly compared to the young group (55.8% vs 40.8%; p= 0.09).
More significant differences were observed in sensitive engagement, with greater involvement in deep sensitivity (72.5% vs 29.6%; p=0.001) and ataxia (30.2% vs 13.2%; p=0.01) in the elderly group with statistical significance.
No relevant differences were observed between the groups for dysautonomias and a tendency to greater commitment of cranial nerve in the group of young patients (39.5% vs 50%; p= 0.2).
Electrodiagnosis [see Table N°2]
Parameters
Adults group 18 - 59 years (n=98)
Elderly group = 60 years (n=43)
P value
Electrophysiology
Primary demuelinating; n (%)
69 (70.4%)
30 (69.8%)
0.7
Axonal; n (%)
12 (12,2%)
4 (9.3%)
0.4
Lumbar cerebrospinal fluid
Albuminocytological dissociation; n (%)
63 (64.2%)
31 (72,1%)
0.1
Table 2: Electrophysiological and laboratory variables.
Electrophysiological information was available on all included patients. There was no evidence of a significant difference in the electrophysiological variants (following Hadden's criteria) of the disease between the groups (demyelinating elderly group: 69.8% vs young group: 70.4%; p=0.7).
Cerebrospinal Fluid [see Table N°2]
Lumbar Cerebrospinal Fluid (CSF) was obtained between days 2 and 35 of the disease (mean: 9.3 days), in 42 patients in the elderly group and 96 patients in the young group. Albuminocytological dissociation (proteins greater than 45 mg/dl and cellularity less than 5 cells/mm3) was observed in a similar proportion between the groups (elderly group: 72.1% vs young group: 64.2%; p=0.1) and the protein value did not present differences between them (median g/dl elderly group: 74 vs young group: 79; p=0.6).
Treatment and Follow-up [see Table N°3]
Variables
Adults group 18 - 59 years (n=98)
Elderly group = 60 years (n=43)
P value
Treatmant
Delay to treatment (median in days)
7
7
0.3
Gammaglobulin; n (%)
9 (9,2%)
2 (4.6%)
0.3
Plasmapheresis; n (%)
90 (91.8%)
39 (90.7%)
0.9
More than 1 treatment; n (%)
3 (3.1%)
2 (4.6%)
0.6
Refractory; n (%)
4 (4.1%)
1 (2.3%)
0.3
Fluctuations; n (%)
5 (5.1%)
3 (7%)
0.6
Days of hospitalization; Median (range)
6 (1 - 52)
7 (4 - 49)
0.4
ICU required; n (%)
16 (16,3%)
3 (7%)
0.2
- Days in ICU Avarege (median)
48,2 (16.5)
29,3 (15)
0.2
- Mechanical respiratory assistance: n (%)
5 (31%)
0
0.3
- Non-invasive ventilation; n (%)
8 (50%)
3 (100%)
0.1
Outcome
Death; n
0
1
Hughes score at 6 months; Avarege (Median)
0.64 (0)
0.69 (0)
0.7
Hughes score at 12 months;Avarege (Median)
0.22 (0)
0.29 (0)
0.6
ICU: Intensive care unit.
Table 3: Treatment and outcome variables.
Concerning the treatment, gamma globulin was mainly used in both groups (elderly group: 90.7% vs young group: 91.8%; p=0.3). In a small proportion, plasmapheresis was performed in the first instance (elderly group 4.6% vs young group 3.1%; p=0.9). In both groups, the delay until the initiation of treatment was a median of 7 days. Exceptionally, the "wait and see" strategy was chosen, only 2 patients in the elderly group and 5 patients in the young group.
The days of hospitalization in both groups were similar, presenting a median of 7 days in the elderly (range 4 - 59 days) and 6 days in young group (range 1 - 52 days) (p=0.4).
Only 3 (7%) elderly patients required ICU monitoring, unlike the young group where 16 (16.3%) of them required it (p=0.2).
Only one elderly patient died, while the young group there were no deaths. In the follow-up of the patients, assessing the degree of disability at 6 and 12 months using the Hughes scale (17), in both groups the evolution was favorable and similar in these values (Hughes average at 6 months elderly group 0.69 vs young group 0.64; p=0.7) (Hughes Average at 12 months elderly group 0.22 vs young group 0.29; p=0.6).
Emphasizing the elderly group, only 2 patients did not show improvement after the established treatment and 2 patients did not recover the ability to walk.
Discussion and Conclusions
Previous reports describe a linear increase in the incidence of GBS with age [2]. Nevertheless, in our population, we observed a bimodal distribution presenting a peak at 60 years of age and a decrease after 80 years of life, similar to that described by other authors in European and Asian populations [5-7].
Analyzing the medical history of the patients, specifically diabetes considering it a risk factor for the presence of previous neuropathy, a tendency to higher incidence was evidenced in the elderly group (13.9% vs 5.1%; p value=0.07). This aspect may be related to the greater compromise of deep sensitivity (72.5% vs 29.6%; p=0.001) and ataxia (30.2% vs 13.2%; p=0.01) in this age group. This is not usually detailed in other works, however, we must be taken into consideration for an adequate approach to rehabilitation.
Furthermore, no significant differences were observed between the groups for the incidence of prodromal events and respiratory infections being the most frequent just like described by Peric et. al [11]. Also, this author describes a mean time from precipitating factor to GBS onset similar between the two groups. Nevertheless, we highlight a longer delay from the prodromal event to the onset of GBS in the elderly with statistical significance (median in days 14 vs 7; p=0.04) (Graph N°1). This could be interpreted as a more insidious presentation in the context of immunosenescence in the elderly.
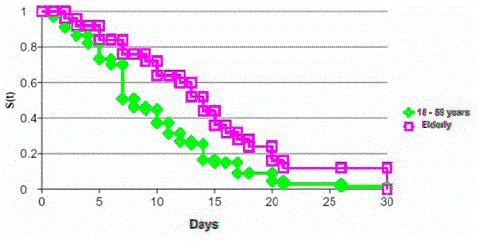
Graph 1: Kaplan meier curve - Time from prodromal to clinical event.
Using Hugges disability scale, we observed a trend of greater presentation of severe GBS (score 3 to 5) in the elderly (55.8% vs 40.8%; p=0.09), the same as that described by Peric et. al. [11], given the degree of motor compromise suffered by these patients. However, in our patients this aspect was not reflected in ICU hospitalization (elderly group 7% vs young group 16.3%; p=0.2) requirements or in mortality (only 1 patient from de elderly group died), unlike what was described by Peric et. al. [11] and Zhang et. al [12]. This may be related to the small number of patients who presented compromised lower cranial nerves or severe dysautonomia.
In the complementary studies, a relationship of higher incidence of axonal variants in the pediatric population compared to adults has been widely described [19]. But, if subgroups of the adult population are compared, the results are contradictory. In all case series the demyelinating variant is predominant, however Peric et. al [11] describe that axonal variants were twice more frequent in the elderly group. In our case this was not found, having a similar proportion between the groups (demyelinating elderly group: 69.8% vs young group: 70.4%; p=0.7), similar to described by Zhen et. al. [12].
In the other hand, albuminocytological dissociation and protein concentration in CSF according to age is controversial in the literature. Some authors even propose changing the threshold for protein rachia based on age, considering it elevated above 60mg/dl from 50 years of age [20]. Bourque et. al. [21] assessed this diagnostic criterion in GBS, without showing a change in the sensitivity of albuminocytological dissociation from the second week after the onset of symptoms. In our series of cases, CSF was obtained predominantly in the second week and observed a similar proportion in both groups for the cytological albumin dissociation and the level of protein.
Finally, in terms of prognosis, in our series of cases it was observed that the elderly had a good response to treatment. This is reflected in an excellent prognosis of motor functionality with a 12-month clinical follow-up (Hugges Average at 12 months elderly group 0.22 vs young group 0.29; p=0.6). Only 2 patients did not recover their ability to walk and only 1 patient died at the nadir of the disease. These results differ from those described by Peric et. al. [11] and Zhen et. al. [12], where advanced age is related to a worse short term prognosis. We consider that this difference could be due to the low frequency of bulbar involvement and severe dysautonomia in our population.
Consequently, with our results we cannot conclude that advanced age is an independent risk factor for a worse prognosis. However, it must be considered that the Latin American population has lower survival compared to the European population, which results in a low number of patients in the elderly group (especially those over 80 years of age). Therefore, a higher “n” would be required to obtain more robust conclusions.
References
- Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barré syndrome. Lancet. 2016; 388: 717-727.
- Sejvar JJ, Baughman AL, Wise M, Morgan OW. Population incidence of Guillain-Barré syndrome: a systematic review and meta-analysis. Neuroepidemiology. 2011; 36: 123-133.
- Blum S, Reddel S, Spies J, McCombe P. Clinical features of patients with Guillain-Barré syndrome at seven hospitals on the East Coast of Australia. J Peripher Nerv Syst. 2013; 18: 316-320.
- Peric S, Milosevic V, Berisavac I, Stojiljkovic O, Beslac-Bumbasirevic L, et al. Clinical and epidemiological features of Guillain-Barré syndrome in the Western Balkans. J Peripher Nerv Syst. 2014; 19: 317-321.
- Cuadrado JI, de Pedro-Cuesta J, Ara JR, Cemillán CA, Díaz M, et al. Guillain-Barré syndrome in Spain, 1985-1997: epidemiological and public health views. Eur Neurol. 2001; 46: 83-91.
- Chroni E, Papapetropoulos S, Gioldasis G, Ellul J, Diamadopoulos N, et al. Guillain-Barré syndrome in Greece: seasonality and other clinico-epidemiological features. Eur J Neurol. 2004; 11: 383-388.
- Chen Y, Ma F, Zhang J, Chu X, Xu Y. Population incidence of Guillain-Barré syndrome in parts of China: three large populations in Jiangsu province, 2008-2010. Eur J Neurol. 2014; 21: 124-129.
- Fujimura H. The Guillain-Barré syndrome. Handb Clin Neurol. 2013; 115: 383-402.
- Verboon C, van Doorn PA, Jacobs BC. Treatment dilemmas in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. 2017; 88: 346-352.
- Devos D, Magot A, Perrier-Boeswillwald J, Fayet G, Leclair-Visonneau L, et al. Guillain-Barré syndrome during childhood: particular clinical and electrophysiological features. Muscle Nerve. 2013; 48: 247-251.
- Peric S, Berisavac I, Stojiljkovic Tamas O, Rajic S, Babic M, et al. Guillain-Barré syndrome in the elderly. J Peripher Nerv Syst. 2016; 21: 105-110.
- Zhang B, Wu X, Shen D, Li T, Li C, et al. The clinical characteristics and short-term prognosis in elderly patients with Guillain-Barré syndrome. Medicine (Baltimore). 2017; 96: e5848.
- França MC Jr, Deus-Silva L, de Castro R, Garibaldi SG, Pfeilsticker BH, et al. Guillain-Barré syndrome in the elderly: clinical, electrophysiological, therapeutic and outcome features. Arq Neuropsiquiatr. 2005; 63: 772-775.
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990; 27: S21-4.
- Merkies IS, Schmitz PI, van der Meché FG, Samijn JP, van Doorn PA. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatry. 2002; 72: 596-601.
- Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice (Fourth Edition). 2013.
- Hadden RD, Cornblath DR, Hughes RA, Zielasek J, Hartung HP, et al. Electrophysiological classification of Guillain-Barré syndrome: clinical associations and outcome. Plasma Exchange/Sandoglobulin Guillain-Barré Syndrome Trial Group. Ann Neurol. 1998; 44: 780-788.
- Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978; 2: 750-753.
- Maximiliano A. Hawkes, Miguel Wilken, Gabriel Vázquez, Mauricio F. Farez. Age may contribute to the increased frequency of axonal Guillain-Barré syndrome. Muscle and nerve. 2017; ;56: 1171-1173.
- Breiner A, Moher D, Brooks J, Cheng W, Hegen H, et al. Adult CSF total protein upper reference limits should be age-partitioned and significantly higher than 0.45 g/L: a systematic review. J Neurol. 2019; 266: 616-624.
- Bourque PR, Brooks J, McCudden CR, Warman-Chardon J, Breiner A. Age matters: Impact of data-driven CSF protein upper reference limits in Guillain-Barré syndrome. Neurol Neuroimmunol Neuroinflamm. 2019; 6: e576.