
Clinical Geriatrics
Gerontol Geriatr Res. 2023; 9(2): 1089.
Statin Use and Venous Thromboembolism Incidence: A Case-Control Study in Patients Aged 75 Years and Older
Lisa Guénard1,2; Aurélie Lafargue² ; Eric Ouattara³; Fabrice Bonnet4,5; Isabelle Bourdel-Marchasson2,6*
1Université de Bordeaux, UFR de Médecine, Bordeaux, France
2CHU de Bordeaux, Pole de gérontologie clinique, Bordeaux, France
3CHU de Bordeaux, Service d’information médicale, UCAIM-DIM, Bordeaux, France
4CHU de Bordeaux, Service de Médecine Interne et Maladies Infectieuses, hôpital Saint-André, CHU de Bordeaux, Bordeaux, France
5ISPED, INSERM U1219, Bordeaux Population Health Research Center, University of Bordeaux, F-33000, Bordeaux, France
6CNRS/Université de Bordeaux, CRMSB, UMR 5536, Bordeaux, France
*Corresponding author: Marchasson IB Centre Henri Choussat, Hôpital Xavier Arnozan, 33604 Pessac, France Email: Isabelle.bourdel-marchasson@chu-bordeaux.fr
Received: May 03, 2023 Accepted: May 27, 2023 Published: June 03, 2023
Abstract
Purpose: Although previous observational and controlled studies have reported a lower rate of venous thromboembolism in statin users, such studies were conducted in selected patients and their results may not be extended to an older multi-morbid population. This study aimed to investigate the influence of statin use on the risk of Venous Thromboembolic Events (VTE) in older patients.
Methods: We conducted a case-control study on patients aged 75 and older, hospitalized in the Medicine or Geriatric Departments from 2009 to 2019. Cases with a documented episode of VTE were included and compared with random controls matched for age, gender, year of hospitalization, and previous statin use. Patients under long-term anticoagulation therapy before admission were excluded.
Results: Of the 177 cases, 31 (17.5%) reported statin use, as well as 36 (20.3%) controls. Statin use was not significantly associated with a lower rate of VTE (Odds Ratio [OR] 0.8 95% Confidence Interval [CI] [0.5-1.4], p=0.5). Adjustments for age, sex, atherosclerotic disease, major risk factors for VTE, and use of antiplatelet agents did not alter the results. Neither aspirin (OR 1.0 95% CI 0.6-1.6], p=1.0) nor other antiplatelet therapies (OR 0.6 95% CI [0.3-1.3], p=0.2) were associated with a reduced rate of VTE. Statin users had significantly more comorbidities, such as atherosclerotic disease, hypertension, and diabetes than non-users.
Conclusion: This case-control study does not support a protective effect of statins on VTE risk in older inpatients.
Keywords: Statin; Venous thromboembolism; Older; Case-control; Comorbidities
Introduction
Venous Thrombo Embolism (VTE), including Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE) is the third leading cause of cardiovascular disease in western countries and is strongly associated with age. The incidence of VTE is 3-4/1,000 per year among those aged 65 years and older, which increases to 1% in the oldest [1,2]. Despite a well-defined therapeutic strategy based on anticoagulation [3] morbidity and mortality related to VTE remain substantial in older patients, and bleeding complications under treatment are more frequent than in younger patients [4-6]. Thus, reinforcing primary and secondary prevention of VTE is of growing interest.
Primary prevention mainly relies on low-dose-heparin and low-molecular-weight-heparin prophylaxis for at-risk situations, yet these therapies do not eliminate the VTE risk [3]. Anticoagulant therapy is effective for preventing both incident and recurrent VTE, and the guidelines recommend extended anticoagulant use after a first VTE episode in patients at high risk of recurrence [3,7,8]. Nevertheless, anticoagulant therapy is associated with bleeding complications that may limit its use in older and multi-morbid patients. Identifying older individuals with a favorable risk-benefit ratio for long-term anticoagulation therapy may be challenging [9,10]. Older adults, who are at higher risk for both VTE and bleeding complications, may benefit from a preventive treatment that would not increase the risk of bleeding.
JUPITER was a randomized controlled interventional trial that investigated the effectiveness of a high potency statin, rosuvastatin 20mg daily, in the primary prevention of vascular events, including VTE. The authors reported a significant 40% risk reduction in the occurrence of VTE in healthy subjects receiving rosuvastatin [11]. This result was consistent with other studies suggesting that the benefits of statin therapy rely not simply on its lipid-lowering effect. Statins may exert pleiotropic effects by reducing inflammatory markers, inhibiting platelet activation, improving endothelial function, and interfering with the blood coagulation system [12]. Thus, the hypothesis of a protective effect of statins in non-atherosclerotic vascular diseases, such as VTE, has been proposed.
Several observational studies have reported a significant decrease in the risk of VTE in statin-treated patients, with different relative risk reductions, ranging from 20% to 60% [13-15]. Most of these studies included young or middle-aged patients with few comorbidities. In the post-hoc analysis of the PROSPER study, the authors found a lack of a protective effect of 40 mg pravastatin daily on first VTE incidence in subjects aged 70-82 years [16]. Whether statin use is associated with a lower risk of VTE in all patient subgroups remains controversial. Furthermore, data in older patients are scarce, even though they represent a high proportion of VTE cases (70% in those aged ≥60 years) [2].
The objective of this case-control study was to evaluate the association between statin use and VTE in hospitalized patients aged 75 years or older presenting with acute VTE.
Methods
Design and settings
This monocentric cross-sectional case-control study compared hospitalized patients aged 75 years and older, presenting with acute symptomatic VTE, according to the use of statins. All patients were hospitalized between 2009 and 2019 in two wards (Internal or Geriatric Medicine).
Our primary objective was to evaluate the association between statin use and the occurrence of VTE. The primary outcome was a comparison of the presence of recent VTE according to statin use in case and control patients who were not undergoing anticoagulation therapy, estimated with odds ratios (ORs) and 95% Confidence Intervals (CIs). The secondary outcomes were to describe VTE cases (VTE location, provoked and idiopathic events, transient risk factors, comorbidities, and co-medications) and to compare statin users to non-users (comorbidities and co-medications).
This protocol was approved by the hospital ethical review board.
Study Population
A list of potential cases and their matched controls were obtained in a request to the medical information department of CHU of Bordeaux. Medical records were extracted from the hospital database using the ICD-10 classification (International Classification of Diseases). The eligibility of all patients was reviewed by the study investigators.
Cases were defined as patients aged 75 years and older diagnosed with symptomatic PE, proximal DVT of the lower limb, or both. The VTE diagnosis had to be confirmed with validated objective imaging methods. DVT was defined as the acute onset of leg pain or swelling, associated with incomplete compressibility of a proximal venous segment on ultrasonography. PE was defined using the association between the clinical symptoms (dyspnea or oxygen requirement, chest pain, hemoptysis, or syncope) and an intraluminal filling defect on contrast spiral Computed Tomography (CT), or a high probability ventilation/perfusion lung scan when CT was contraindicated.
A control subject matched to the same age, gender, and hospitalization year was randomly assigned to each case subject. Cases and their matched controls had to be hospitalized during the same year in one of the participating units. Controls were hospitalized patients who were not diagnosed with VTE.
The exclusion criteria for cases were venous thrombosis at an atypical location (cerebral, portal, jugular, or inferior or superior vena cava thrombosis), DVT of the upper limbs, isolated distal DVT of the lower limb, and superficial venous thrombosis. Controls were excluded if they had had a previous episode of objectively confirmed VTE. Cases and controls were excluded if they had been treated with anticoagulant therapy before admission.
Data Collection
Baseline data were collected from the hospital database by the study investigator, including demographic information (age and gender), comorbid conditions (history of DVT, myocardial infarction or angina pectoris, congestive heart failure, peripheral vascular disease, cerebrovascular accident or transient ischemic attack, hypertension, chronic pulmonary disease, liver disease, renal insufficiency, diabetes, cancer, and dementia). The Charlson Comorbidity Index was calculated for each patient [17].
The use of medication at the time of diagnosis was recorded: statin (drug and dose), other lipid-lowering drugs, oral antiplatelet therapy, antihypertensive therapy, oral anti-diabetic and insulin therapy, psychotropic medications, and cancer treatment, such as current chemotherapy, radiotherapy, or hormone therapy.
Biological data were recorded at the time of hospital admission, including plasma creatinine (μg/l) and C-reactive protein (mg/l) levels. Creatinine clearance was calculated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) formula. Transient risk factors for VTE were recorded and included surgery within the past 3 months, fracture of a lower limb within the past 3 months, recent immobilization (defined as =72 hours nonsurgical bedridden status in the past 15 days), cancer (active cancer, under treatment or not, or cancer in remission for less than 6 months), and severe infection [18]. Cases without any transient risk factors were considered to have idiopathic VTE.
Statin use was defined as the use of statin therapy at the time of hospital admission.
Statistical Analysis
The null hypothesis was the prevalence of VTE is equal or superior in patients who use statins compared to patients who do not. The required number of subjects to test this hypothesis unilaterally, with a ratio of one control for one case, with a statistical power of 85% and an alpha risk of 0.05, was estimated from a previous case-control study [13]. In the latest study that included 377 patients, the OR for VTE was 0.42 in statin users compared to non-users. In this study, a minimal detectable OR of 0.45 was chosen to detect a difference and a large effect size, which may be more likely to have practical significance. The prevalence of statin use was tailored according to recent observational studies [19] and the usually observed prevalence in geriatrics wards, resulting in an expected prevalence of statin use in older patients of 20%. Thus, we obtained the required 332 subjects (166 in the case group and 166 in the control group).
The characteristics of the cases and controls were compared using the chi-square test for categorical data or Fisher’s exact test for paired values, when appropriate. Continuous variables were compared with Student’s t-test for paired values. The OR for VTE was estimated according to drug use in the matched cases and controls.
A pre-planned stratified analysis was performed to explore potential confounders known to be either risk factors or protective factors for VTE, including age, sex, transient major risk factors, cardiac insufficiency, coronary heart disease, cerebrovascular disease, occlusive arterial disease of the lower limb, chronic lung disease, and exposure to antiplatelet therapy (aspirin, clopidogrel, or ticagrelor). Homogeneity of the data was assessed using the Breslow-Day test, and adjusted ORs were calculated with the Mantel-Haenszel method.
The comparisons of statin users and non-users were pre-planned and performed with the chi-square and Fisher’s exact test for categorical variables and Student’s t-test for paired continuous variables. The data analysis was conducted with SPSS© software (SPSS version 23; SPSS Inc., Chicago, IL, USA). A p-value < 0.05 was considered significant.
Results
Participants
In total, 354 patients were enrolled (177 cases and 177 controls; Figure 1). All patients had been hospitalized between 2009 and 2019 at the University Hospital in Bordeaux. The baseline characteristics of the participants are summarized in Table 1.
Variables
Cases, n=177
Controls, n=177
p-value
Age, years (mean±standard deviation)
86±6
86±6
0.97
Sex [women, n(%)]
112 (63)
112 (63)
1
Proximal deep vein thrombosis-DVT (n)
77
Pulmonary embolism-PE (n)
136
DVT+PE (n)
36
Presence of major risk factors for venous thromboembolism-VTE×[n(%)]
156 (88)
135 (77)
0.05
Prior VTE [n(%)]a,
33 (19)
-
Coronary heart disease [n(%)]a,
35 (20)
26 (15)
0.26
Cardiac insufficiency [n(%)]a,
40 (22)
44 (25)
0.71
Cerebrovascular disease [n(%)]b,
57 (32)
51 (29)
0.56
Occlusive peripheral arterial disease [n(%)]c,
32 (31)
34 (33)
0.9
Chronic lung disease [n(%)]d,
35 (20)
30 (17)
0.58
Diabetes [n(%)]a,
30 (17)
26 (15)
0.6
Body mass index, kg/m2 (mean, SD)e,
25±6
24±5
0.4
Cognitive troubles [n (%)]f,
93 (73)
81 (65)
0.24
Creatinine clearance, estimated with the CKD-EPI formula, (ml/min, mean, SD)g,
61±22
61±23
0.8
Active cancer, [n(%)]
48 (27)
20 (11)
0.002
Myeloproliferative syndrome, [n(%)]
0
2 (1)
0.5
Lymphoma, [n(%)]
1 (0,5)
3 (2)
0.62
Leukemia, [n(%)]
3 (1,5)
1 (0,5)
0.62
Charlson comorbidity index (mean, SD)
9±3
8±3
0.002
SD: standard deviation.
*Secondary VTE defined as major surgery within the past 3 months, fracture of a lower limb within the past 3 months, immobilization=72 hours within the past 15 days, or active cancer. A Data available for 177 cases and 175 controls. B Data available for 174 cases and 174 controls. C Data available for 103 cases and 104 controls. D Data available for 177 cases and 174 controls. E Data available for 127 cases and 124 controls.
F Data available for 138 cases and 124 controls. G Data available for 156 cases and 135 controls.
Table 1: Baseline demographic and clinical characteristics of the study population (n=354).
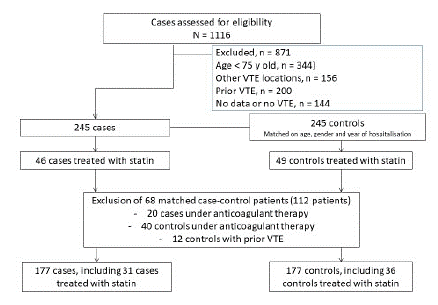
Figure 1: Flow diagram of patients in the study.
A total of 63% (n=112) of the participants in both groups were women. The mean age of the study population was 86 years (range 75-102 years). The majority of participants had comorbidities, such as cardiac insufficiency, atherosclerotic disease, chronic lung disease, chronic renal disease, and cognitive impairment, resulting in a high Charlson comorbidity index score in both groups. Among the cases, 44 had an isolated proximal DVT, 100 had an isolated PE, and 36 had PE associated with DVT. A total of 19% (n=22) of cases had had a previous episode of VTE. Major risk factors for VTE (surgery within the past 3 months, fracture of a lower limb within the past 3 months, immobilization ≥72 hours within the past 15 days, or active cancer) were present at the time of admission in most patients (88% in in the case group, 77% in the control group, p=0.05). Significantly more active cancers were observed among the cases (27% [n=48] of patients in the case group vs. 11% [n=20] in the control group; p=0.002). The prevalence of atherosclerotic disease, cardiac insufficiency, chronic lung disease, renal insufficiency, diabetes, and cognitive disorders did not differ significantly between the groups. The mean Charlson Comorbidity Index score was 1 point higher in the case group than in the controls (9±3 in cases vs. 8±3 in controls, p=0.002).
Comparison of VTE risk according to statin use
In total, 67 patients were using a statin at the time of admission, including 24 atorvastatin, 19 simvastatin, 12 rosuvastatin, 11 pravastatin, and one fluvastatin. Other lipid-lowering treatments were found in 18 patients: nine were taking fenofibrate, two were taking bezafibrate, one was taking ciprofibrate, and two were taking ezetimibe alone. Four patients were using simvastatin and ezetimibe. Statin use was not associated with a lower prevalence of VTE (OR 0.8, 95% CI [0.5-1.4], p = 0.5) (Table 2). Other lipid-lowering therapies and antiplatelet agents were not associated with a lower rate of VTE (Table 2).
Drug exposure
Number of exposed cases
Number of exposed controls
Odds Ratios
p-value
(95% CI)
Statin
31
36
0.8 [0.5-1.4]
0.5
Other lipid-lowering therapies
8
10
0.9 [0.3-2.3]
0.8
Aspirin
58
58
1.0 [0.6-1.6]
1
Other antiplatelet therapies (clopidogrel or ticagrelor)
10
16
0.6 [0.3-1.3]
0.2
Table 2: Matched odds ratios (ORs) and 95% Confidence Intervals (CIs) for venous thromboembolism related to lipid lowering and anti-platelet therapies.
A stratified analysis of potential confounders did not reveal any significant interactions, with similar crude and adjusted ORs (Table 3). Cancer status between the cases and controls was heterogeneous. Thus, the effect of cancer could not be investigated.
Strata
Number of exposed cases
Number of exposed controls
Adjusted odds ratios
P-value
[95% IC]
Major VTE risk factors*
Present*
28/156
26/134
2.2 [1.3-3.9]
0.45
Absent*
21-May
Aug-41
Aspirin <300 mg/daily
Users
Nov-58
19/58
1.1 [0.6-1.6]
0.09
Non-users
20/118
17/118
Other antiplatelet drugs
Users
10-May
16-Jul
0.6 [0.3-1.4]
0.6
Non-users
26/166
29/159
Heart failure
Present
Oct-40
Nov-44
0.8 [0.5-1.4]
0.6
Absent
21/137
25/131
Coronary heart disease
Present
14/35
26-Oct
1.6 [0.9-2.9]
0.5
Absent
17/142
26/149
Cerebrovascular disease
Present
Oct-51
Nov-57
0.8 [0.5-1.3]
0.9
Absent
22/123
24/117
Occlusive peripheral arterial diseasea
Present
Sep-32
13/34
0.9 [0.5-1.8]
0.8
Absent
Oct-71
Dec-70
Chronic lung disease
Present
Jun-35
30-Jul
1.2 [0.7-2.1]
0.7
Absent
25/142
29/144
Sex
Female
18/111
23/112
0.9 [0.6-1.5]
0.6
Male
13/68
13/63
Age
>80 years
24/144
28/144
1.0 [0.6-1.7]
0.9
=80 years
Jul-32
Aug-32
>90 years
Apr-37
Apr-37
1.0 [0.6-1.7]
0.8
=90 years
27/139
27/139
Abbreviations: 95% CI, 95% confidence interval; VTE, venous thromboembolism. P-values were calculated with the Breslow-Day test for homogeneity and adjusted odd ratios were determined with the Mantel-Haenszel method. * Major VTE risk factors included major surgery within the past 3 months, fracture of a lower limb within the past 3 months, immobilization=72 hours within the past 15 days, or active cancer. a Data available for 103 patients in the case group and 104 patients in the control group.
Table 3: Use of statins and VTE risk: stratified analysis of potential risk and protective factors.
Comparison of statin users and non-users
Table 4 presents a comparison of statin users and non-users according to their comorbidities and co-medications by antiplatelet therapy. Statin users were younger, had more coronary heart disease (35% vs. 13%, p=4.2 10-5), hypertension (86% vs. 69%, p=0.016), occlusive peripheral arterial disease (50% vs. 28%, p=0.006), and diabetes (25% vs. 4%, p=0.024) than non-users. The Charlson Comorbidity Index values were not normally distributed and were compared with the non-parametric Mann-Whitney test. The results were not significantly different between the two groups (9±3 vs. 8±3, p=0.12).
Variables
Statin
Statin
p-value
users,
non-users, n = 287
n=67
Age (years, mean±SD)**
84±5
87±6
0.025
Sex [women, n(%)]*
42 (62)
186 (64)
0.77
Previous episode of VTE [n(%)]*
8 (12)
25 (9)
0.83
Coronary heart disease [n(%)]*
24 (35)
37 (13)
4.2 10-5
Heart failure [n(%)]*
21 (31)
63 (22)
0.11
Cerebrovascular disease [n(%)]*
20 (30)
88 (31)
1
Occlusive peripheral arterial disease[n(%)]*a,
22 (50)
45 (28)
0.006
Hypertension [n(%)]*
59 (86)
198 (69)
0.016
Chronic lung disease [n(%)]*
13 (19)
53 (19)
0.86
Diabetes [n(%)]*
17 (25)
39 (14)
0.024
Body mass index, kg/m2 (mean±standard deviation)**
26±5
24±6
0.072
Creatinine clearance, estimated by the CKD-EPI formula (ml/min, mean±SD)**
57±24
58±23
0.13
Cognitive troubles [n(%)]*
23 (56)
151 (71)
0.009
Active cancer, [n(%)]*
15 (22)
53 (18)
0.49
Charlson comorbidity index (mean±SD)***
9±3
8±3
0.12
Aspirin*
30 (44)
85 (30)
0.02
Other antiplatelet therapy*
12 (18)
14 (5)
0.008
Abbreviations: VTE: Venous Thrombo Embolism; SD: Standard Deviation. A Data available for 44 of 67 statin users and 165 of 288 non-users.
Table 4: Statin user and non-user characteristics.
Discussion
Statin use was not associated with a reduced rate of VTE in very old and multi-morbid hospitalized patients. This finding differs from several studies in which the risk of the occurrence of VTE decreased in statin users [11,13-15]. Nevertheless, the study population of the JUPITER trial [11] was designed to assess the effect of statins on the primary prevention of vascular events. The participants were younger and less comorbid, as they excluded patients with a history of cardiovascular disease, diabetes, and cancer. In a above-cited previous French case-control study, patients were also younger (mean age 68 years) and healthier [13]. The post-hoc analysis of the PROSPER trial, which evaluated statin use and primary prevention of older adults, included patients aged 70-82 years with comorbid conditions and vascular risk factors and did not find a protective effect of 40 mg pravastatin daily on VTE risk. In this later study, patients with cancers were not excluded and cancers were more prevalent in patients with VTE. Adjusting for cancer status did not alter their results [16]. Older age and comorbidities may strongly attenuate the potential protective effect of statins on VTE.
In the present study, a large majority of patients had a provoked VTE related to major transient or permanent risk factors. These risk factors were more prevalent in the case group than the control group. Most previous studies reported an association between statins use and a lower incidence of idiopathic VTE [11,13,20], whereas other authors showed a weaker protective effect of statins on provoked VTE compared with idiopathic VTE [21]. We assumed that statins exert a differential effect in provoked vs. idiopathic VTE, but further studies are needed to assess this issue.
Data concerning statin therapy and VTE risk in patients with cancer are conflicting. In an observational prospective cohort, statin use was associated with a 55% decrease in VTE risk in patients with various malignancies. The 1-year cumulative incidence rate was 2.94% in statin users compared to 7.13% in non-users [22]. The benefit appeared to be weaker in another report [23]. The multiplicity of mechanisms implicated in cancer-related thrombosis may be an explanation for the lack of a protective effect of statin therapy in these patients, so further studies are required.
In the general population, VTE occurs as an isolated DVT in two-thirds of cases, and as PE in one-third of cases (isolated or associated with DVT). PE is the most frequent presentation with advancing age [1]. In this study, approximately 75% of the cases developed PE, either isolated or associated with proximal DVT. These results reflect the higher prevalence of PE in old patients, but also the fact that isolated DVT with no complications is more likely to be treated in out-patient care. In the JUPITER cohort, Glynn et al. Reported a protective effect of statins on VTE but this result was mainly driven by the relative risk reduction in DVT, whereas significance was not reached for PE [11]. Statins may exert a protective effect on DVT only. Considering that PE was the main presentation of VTE in the present study, it could explain the observed lack of a protective effect as described in other studies.
This study had several limitations. The cross-sectional design allowed the inclusion of a large number of cases, but is associated with a poor level of evidence and causality cannot be stated. Additionally, the case and control groups were not comparable regarding cancer prevalence. As there is no consensus on the effect of statin use on VTE risk in cancer patients, it is not possible to assess whether this difference led to an under or overestimate in our study. Nevertheless, the stratified analysis did not reveal a significant interaction after adjusting for malignancy or a second analysis that excluded individuals with cancer (data not shown). Third, we were unable to investigate the effect of each drug, dose, and duration of use due to the relatively small number of statin users and the cross-sectional design. Fourth, we measured the primary outcome without considering the incident or recurrent nature of the VTE event. Thus, we cannot draw any conclusions. Finally, the comparison of statin users and non-users revealed that statin users were significantly more often under antiplatelet therapy. This difference may have interfered with the VTE risk, as several studies have reported a protective effect of antiplatelet drugs on venous thrombosis [13,24,25]. Nevertheless, no reduction in VTE prevalence was detected in the matched case-control comparison, according to the use of antiplatelet therapy, and the stratified analysis including protective factors did not show a significant interaction with aspirin or other antiplatelet drugs. Not excluding individuals treated with antiplatelet therapy probably better reflects real-life; an association between statins and aspirin usually corresponds to the presence of atherosclerotic diseases. Excluding these patients may have favored a healthy-user effect.
One strength of this study is the inclusion of a very old and multi-morbid population, usually excluded or under-represented in clinical trials. Yet, as they are at risk of both thrombosis and bleeding under anticoagulant treatment [6] they would have been likely to benefit from a protective effect of statins on VTE if it was confirmed. Our study supports the lack of a preventive effect in this subgroup of patients. We collected data on the presence or absence of major VTE risk factors at the time of admission, which informed us about the provoked or idiopathic nature of each event. Additionally, we selected only proximal DVT and PE, as other locations may have different pathophysiologic pathways and different prognoses. Last, it was decided to exclude individuals treated with anticoagulant therapy, to avoid such a strong confounding factor. To conclude, statin use was not associated with a reduced prevalence of VTE in older hospitalized subjects. The higher prevalence of cancer in the case group may have interfered with this result. The results of this study do not support the use of statins in the preventive strategy of venous thrombophlebitis in the elderly.
Author Statements
The paper was presented at EUGMS virtual meeting in October 2019.
Competing Interests
This study received no funding
The authors: Lisa Guenard (LG), Aurélie Lafargue (AL), Eric Ouattara (EO), Fabrice Bonnet (FB), Isabelle Bourdel-Marchasson (IBM) declare no conflicts of interest.
Author Contribution
Guénard L, Lafargue A and Bourdel-Marchasson I conceived the present idea.
Guénard L, Lafargue A, Ouattara E and Bourdel-Marchasson I had contributed to the design of the present study.
Quality assessment was judged by Lafargue A.
Data collection was realized by Guénard L and Ouattara E.
Analysis and interpretation was made by Guénard L and Lafargue A.
Guénard L, Lafargue A, Ouattara E, Bonnet F and Bourdel-Marchasson I, contributed to critical writing.
Guénard L, Lafargue A, Ouattara E, Bonnet F and Bourdel-Marchasson I approved the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the integrity of any part of the work are appropriately investigated and resolved.
Key Summary Points
Aim: Statin use may prevent thromboembolism event but the evidences in older population are lacking,
Findings: This university-hospital based study did not confirm the potential protective effect of statin use on venous thromboembolism events in older people with high level of comorbidity.
Message: The results of this study do not support the use of statins in the preventive strategy of venous thrombophlebitis in the older patients.
References
- Oger E. Incidence of venous thromboembolism: a community-based study in Western France. EPI-GETBP Study Group. Groupe d’Etude de la Thrombose de Bretagne Occidentale. Thrombosis and haemostasis. 2000; 83: 657-660.
- Rosendaal FR, VANHV A, Doggen CJ. Venous thrombosis in the elderly. Journal of thrombosis and haemostasis: JTH. 2007; 5: 310-317.
- Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). European heart journal. 2020; 41: 543-603.
- Faller N, Limacher A, Méan M, Righini M, Aschwanden M, et al. Predictors and Causes of Long-Term Mortality in Elderly Patients with Acute Venous Thromboembolism: A Prospective Cohort Study. The American journal of medicine. 2017; 130: 198-206.
- Nieto JA, Solano R, Ruiz-Ribó MD, Ruiz-Gimenez N, Prandoni P, et al. Fatal bleeding in patients receiving anticoagulant therapy for venous thromboembolism: findings from the RIETE registry. Journal of thrombosis and haemostasis: JTH. 2010; 8: 1216-1222.
- Spencer FA, Gurwitz JH, Schulman S, Linkins LA, Crowther MA, et al. Venous thromboembolism in older adults: A community-based study. The American journal of medicine. 2014; 127: 530-537.
- Agnelli G, Buller HR, Cohen A, Curto M, Gallus AS, et al. Apixaban for extended treatment of venous thromboembolism. The New England journal of medicine. 2013; 368: 699-708.
- Weitz JI, Lensing AWA, Prins MH, Bauersachs R, Beyer-Westendorf J, et al. Rivaroxaban or Aspirin for Extended Treatment of Venous Thromboembolism. The New England journal of medicine. 2017; 376: 1211-1222.
- Scherz N, Méan M, Limacher A, Righini M, Jaeger K, et al. Prospective, multicenter validation of prediction scores for major bleeding in elderly patients with venous thromboembolism. Journal of thrombosis and haemostasis: JTH. 2013; 11: 435-443.
- Seiler E, Limacher A, Mean M, Beer HJ, Osterwalder J, et al. Derivation and validation of a novel bleeding risk score for elderly patients with venous thromboembolism on extended anticoagulation. Thrombosis and haemostasis. 2017; 117.
- Glynn RJ, Danielson E, Fonseca FA, Genest J, Gotto AM, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. The New England journal of medicine. 2009; 360: 1851-1861.
- Oesterle A, Laufs U, Liao JK. Pleiotropic Effects of Statins on the Cardiovascular System. Circulation research. 2017; 120: 229-243.
- Lacut K, Oger E, Le Gal G, Couturaud F, Louis S, et al. Statins but not fibrates are associated with a reduced risk of venous thromboembolism: a hospital-based case-control study. Fundamental & clinical pharmacology. 2004; 18: 477-482.
- Lassila R, Jula A, Pitkäniemi J, Haukka J. The association of statin use with reduced incidence of venous thromboembolism: a population-based cohort study. BMJ open. 2014; 4: e005862.
- Li R, Yuan M, Yu S, Fu W, Yu W, et al. Effect of statins on the risk of recurrent venous thromboembolism: A systematic review and meta-analysis. Pharmacological research. 2021; 165: 105413.
- Freeman DJ, Robertson M, Brown EA, Rumley A, Tobias ES, Frölich M, et al. Incident venous thromboembolic events in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER). BMC geriatrics. 2011; 11: 8.
- Charlson ME, Pompei P, Ales KL, Mac Kenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987; 40: 373-383.
- Anderson FA, Jr., Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003; 107: I9-16.
- Sundvall H, Fastbom J, Wallerstedt SM, Vitols S. Use of statins in the elderly according to age and indication-a cross-sectional population-based register study. European journal of clinical pharmacology. 2019; 75: 959-967.
- Kronenberg RM, Beglinger S, Stalder O, Méan M, Limacher A, et al. Statin therapy and recurrent venous thromboembolism in the elderly: a prospective cohort study. Scientific reports. 2019; 9: 14804.
- Schmidt M, Cannegieter SC, Johannesdottir SA, Dekkers OM, Horváth-Puhó E, et al. Statin use and venous thromboembolism recurrence: a combined nationwide cohort and nested case-control study. Journal of thrombosis and haemostasis: JTH. 2014; 12: 1207-1215.
- Lötsch F, Königsbrügge O, Posch F, Zielinski C, Pabinger I, et al. Statins are associated with low risk of venous thromboembolism in patients with cancer: a prospective and observational cohort study. Thrombosis research. 2014; 134: 1008-1013.
- El-Refai SM, Black EP, Adams VR, Talbert JC, Brown JD. Statin use and venous thromboembolism in cancer: A large, active comparator, propensity score matched cohort study. Thrombosis research. 2017; 158: 49-58.
- Becattini C, Agnelli G, Schenone A, Eichinger S, Bucherini E, et al. Aspirin for preventing the recurrence of venous thromboembolism. The New England journal of medicine. 2012; 366: 1959-1967.
- Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, et al. Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation. 2014; 130: 1062-1071.