
Special Article: Disability and Rehabilitation
Gerontol Geriatr Res. 2023; 9(3): 1094.
Cognitive Sequelae, Autonomy, Social Participation and Quality of Life Over 10 Years after Traumatic Brain Injury
Holin S1*; Allart E2,3; Kozlowski O3-5; Delecroix H5; Moroni C1
1Univ. Lille, ULR 4072 – PSITEC - Psychology: Interactions, Time, Emotions, Cognition, France
2Univ. Lille, Inserm UMR-S-1172 – Lille Neuroscience and Cognition, Univ. Lille, France
3Neurorehabilitation unit, Lille University Hospital, France
4Reseau de la cérébrolésion TC-AVC Hauts-de-France, France
5Auprès-TC, Fondation Partage et Vie, France
*Corresponding author: Samantha Holin Univ. Lille, ULR 4072 – PSITEC - Psychology: Interactions, Time, Emotions, Cognition, 59000 Lille, France. Email holin.sam@gmail.com
Received: June 28, 2023 Accepted: August 10, 2023 Published: August 17, 2023
Abstract
Objective: This study aimed to describe the cognitive status, autonomy, quality of life, and social participation of individuals who had experienced a Traumatic Brain Injury (TBI) at least 10 years prior.
Method: Data were collected from 29 individuals with moderate to severe TBI, with a mean age of 49 at the assessment time. Interviews were conducted approximately 22 years after the onset of the brain injury (mean age at onset was 27 years). Participants were divided into two groups based on the time elapsed since the TBI. Participants were asked about changes in their difficulties with age.
Results: The study revealed that half of the participants perceived a decline in their cognitive abilities. Autonomy in activities of daily living deteriorated more than basic autonomy. Quality of life appeared to improve with age. Furthermore, there was a correlation between social participation and overall cognitive ability. No differences were found between the participant groups, suggesting that the time elapsed since the TBI did not seem to influence their progression.
Conclusion: These findings underscore the evolution of the abilities of individuals with TBI several years after the initial incident, emphasizing the importance of long-term follow-up to tailor support throughout the individual’s lifespan. The study also demonstrates substantial variability in developmental profiles. Additionally, social participation emerges as a pivotal factor to consider, potentially mitigating cognitive decline as individual’s age.
Keywords: Traumatic brain injury; Aging; Cognitive abilities; Autonomy; Quality of life; Social participation.
Abbreviations: DANEL: Dépistage Autonomie du Nord Et du Littoral; MoCA: Montreal Cognitive Assessment; PART-O: Participation Assessment With Recombined Tools–Objective; QOLBI: Quality of Life after Brain Injury; QOLIBRI: Quality Of Life after traumatic Brain Injury; TBI: Traumatic Brain Injury
Introduction
Traumatic Brain Injury (TBI) is part of the most common medical conditions and is the leading cause of acquired disability in individuals aged 15 to 30 [1] as it is responsible for cognitive and behavioral disorders. Cognitively, there is an impairment of mnemonic, executive, and attentional functions, as well as a slowdown in cognitive processing speed. Behaviorally, individuals who have experienced a TBI exhibit fatigue, irritability, frustration intolerance, and even apathy [2,3]. The intensity of these cognitive-behavioral sequelae decreases during the first years following the TBI (mainly due to mechanisms of brain plasticity and recovery), after which they stabilize [4,5]. For 22.2% of patients, this improvement in performance can continue for up to five years after the TBI [6-10]. However, in the medium term (five years after the TBI), these cognitive-behavioral difficulties impact the independence of individuals with moderate to severe TBI, especially for complex activities (cooking, shopping, managing finances, administrative tasks, etc.) as well as basic activities (personal hygiene, dressing, etc.) of daily life [11]. These cognitive-behavioral challenges lead to a decrease in independence and difficulties in social and occupational reintegration. In this regard, they result in an invisible disability that becomes apparent only in specific situations, such as during professional activities. Therefore, individuals who have experienced a TBI will age while dealing with a disability.
In the aging process of individuals who have not experienced a TBI, cognitive decline is observed, characterized by changes in memory, attention, visuospatial abilities, language, and executive functions [12,13]. According to the "Daily Life and Health" survey of people over 60 years old, 26% of them reported at least one functional limitation (physical, sensory, or cognitive), 12% had difficulty bathing (basic autonomy decline), and 28% required human assistance for daily activities [14]. A decrease in quality of life has also been demonstrated, which is associated with an increased risk of depression as people age [15]. The existing similarities between the evolution of cognitive-behavioral sequelae after a TBI and the cognitive-behavioral changes described during aging suggest that the aging process for TBI survivors should have specific characteristics. Few studies have focused on the very long-term evolution of cognitive-behavioral sequelae in individuals with moderate to severe TBI. However, some research has investigated this evolution five years after the TBI [6-10]. These studies have shown heterogeneity in long-term cognitive performance after TBI (16 to 30 years), with some individuals improving, others plateauing, and still others experiencing cognitive decline [16,17]. According to the literature review conducted by Wood in 2017, a moderate to severe head injury depletes an individual's cognitive resources, thereby accelerating cognitive decline and potentially leading to premature cognitive aging and an increased risk of dementia. This risk is identified in the literature [18,19]. Moreover, factors such as gender [20], duration of the initial loss of consciousness [21], TBI severity (severe or moderate [22], and advanced chronological age at the time of the TBI [22,23] significantly increase this risk.
Recently, Hicks et al. (2021) [24] through a longitudinal study, demonstrated that 10 years after their TBI, individuals with TBI exhibited poorer cognitive performance compared to a group of healthy volunteers matched in terms of age, gender, and education level. This result confirms the cognitive sequelae following TBI. However, a comparison of cognitive performance for TBI individuals over a 13-year interval did not show a cognitive decline in these individuals. Therefore, individuals with TBI have cognitive performance impacted by the TBI compared to non-brain-injured participants, but with advancing age, their cognitive performance does not decline faster than that of non-brain-injured participants. The study by Hicks et al.[24] suggests that cognitive aging in individuals who have experienced a TBI is not characterized by accelerated cognitive decline.
Currently, there are still few studies in the literature that definitively establish whether accelerated cognitive decline occurs after a TBI. Additionally, Wood (2017) emphasizes the need for studies on the aging process in individuals who have experienced a TBI. The limited existing studies primarily focus on cognitive functioning. In the context of this study, we aim to investigate the evolution of cognitive performance in individuals with moderate and severe TBI as they age and to relate this evolution to their independence, quality of life, and social participation. Social participation is a factor described in studies on physiological aging as associated with successful aging [25]. Thus, individuals who maintain strong social engagement are less likely to experience cognitive decline as they age. We intend to describe the existing connections between cognitive performance, quality of life, and independence in aging individuals with TBI. Is the evolution of these three dimensions the same, and what role does social participation play in this evolution? Does the time elapsed since the TBI influence long-term outcomes?
Materials and Methods
Participants
We selected individuals from the archives of the TC-AVC network in Hauts-de-France (France) who were victims of a TBI meeting the selection criteria: (1) having suffered a moderate to severe TBI (initial Glasgow Coma Scale score =12), (2) being between 18 and 55 years old at the time of the TBI, (3) being at least 10 years post-TBI, (4) having a strong command of the French language, and (5) not having any other neurological history. The inclusion diagram is depicted in Figure 1. Twenty-nine participants took part (22 males and 7 females), with an average age of 49 years (SD=12.4; Min=30 – Max=73), and they experienced a TBI at an average age of 27 years (SD=9.06; Min=18 – Max=51). On average, they were at a distance of 22 years (SD=9.32; Min=10 – Max=47) from their TBI (Figure 1).
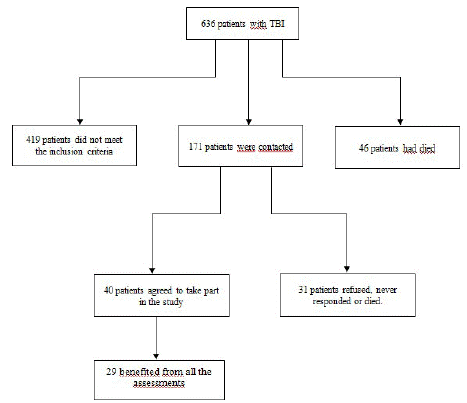
Figure 1: Recruitment Flowchart of Participating Individuals.
In our sample, 3/29 individuals had suffered a moderate TBI, while 26 had experienced a severe TBI. Thirteen participants had a socio-cultural level equivalent to at least a high school diploma (baccalaureate). The majority of participants had multiple focal lesions (26 out of 29), with only three presenting a single focal lesion. At the time of data collection, seven individuals were still being monitored for their TBI, five were living in medical-social facilities, eight were living alone, and 16 were living with their family (either as a couple or with their parents).
The study obtained approval from the South-West and Overseas IV Regional Committee for the Protection of Persons (reference: 2021-A02367-34). All participants (or their legal guardians) provided informed consent.
Procedure
Subjective and objective measures were conducted to assess cognitive abilities, social participation, autonomy, and quality of life. Among our participants, 26 had a complete score on the MoCA test. They were divided into two independent groups based on the time elapsed since the TBI. For 12 of the participants, the data collection occurred between 10 and 18 years post-TBI. Fourteen of them were at 19 years or more (Table 1). The two groups were matched based on age, gender, socio-cultural level (Table 1), age of TBI onset, and TBI severity.
Time elapsed since the TBI
Between 10 and 18 years old N=12
Between 19 and 47 years old N=14
p
Age (M±SD)
42.8 ± 10.5
54.4 ± 12
0.006
Sex (%)
Woman
3 (25%)
3 (21%)
0.86
Man
9 (75%)
11 (79%)
Education level (%)
<high school lever
9 (75%)
6 (43%)
0.111
=high school lever
3 (25%)
8 (57%)
Age of TBI (M±SD)
28.3 ± 9.78
27.4 ± 9.19
0.82
Severity of TBI (%)
Moderate
1 (8%)
2 (14%)
0.652
Severe
11 (92%)
12 (86%)
Life space
Family
8 (66%)
7 (50%)
Alone
2 (17%)
5 (36%)
In medico-social structures
2 (17%)
2 (14%)
Table 1: Demographic data of the 2 groups of participants were distributed according to the time elapsed since the TBI at the time of data collection.
Measures
Subjective assessment of the evolution of the studied parameters: During this interview, we questioned the participants about the difficulties they experienced at 2 years post-TBI (T1) when the post-TBI sequelae had stabilized [4], about the difficulties they were currently facing (T2) and to assess the changes between T1 and T2. For this assessment, a seven-point Likert scale ranging from -3 for a significant deterioration to +3 for a significant improvement was used. Two domains were explored: their cognitive abilities (working memory, episodic memory, attention, inhibition, planning, and mental flexibility) and their quality of life.
Quantitative assessments of cognitive functions, autonomy, social participation, and quality of life
Cognitive functions : In addition to a subjective evaluation, we conducted an objective assessment at T2 using the Montreal Cognitive Assessment (MoCA) testassessment test that is the most sensitive and comprehensive tool for evaluating cognitive functions including attention, concentration, executive functions, memory, language, visuo-constructive abilities, abstraction, calculation, and orientation. A score below 26 (25 for individuals with a cultural level not exceeding a primary school diploma or Certificate of Primary Education) is considered abnormal.
Autonomy
Autonomy was measured using the DANEL questionnaire (Dépistage Autonomie du Nord Et du Littoral) [27]. The DANEL scale is a questionnaire that can be filled out by a caregiver and focuses on both basic and complex autonomy in daily life. The respondent must choose the most appropriate answer from four degrees of autonomy: (A) performs the task independently without being asked; (B) performs the task independently without being asked, but with modified autonomy (slowness, discomfort, fatigue...); (C) performs the task independently when asked to do so; (D) requires the presence of another person to complete the task. A fifth option is also available: (E) Never perform this activity because the person never needs to do it. For example, for the question "cooking," if the person never performs this activity at home because their partner has always taken on this task, the respondent can select option E. In total, a score of 48 is obtained for complex autonomy and 18 for basic autonomy. A higher score indicates lower autonomy. A percentage of autonomy loss can then be calculated, either with or without considering the "never performs this activity" responses (E).
Social Participation
To measure the social participation of TBI individuals, we utilized the adapted 17-item version [28] of the Participation Assessment With Recombined Tools–Objective (PART-O) questionnaire [29] which we validated in the French language. This scale calculates four indices of social participation: (1) productivity (based on time spent in work-related activities, school activities, and household chores), (2) social relations (based on time spent in face-to-face or phone interactions, using instant messaging, having a close friend to confide in, or being engaged in a romantic or sexual relationship), (3) out and about (based on the frequency of activities such as going to restaurants, cinemas, shopping, engaging in physical activities, etc.), and (4) a total participation score (represented by the average of the three sub-scores). Each score can range from 0 to 5. The closer the score is to 5, the higher the level of participation.
Quality of Life
Quality of life was assessed using a questionnaire constructed based on common questions from the QOLBI (Quality of Life after Brain Injury) [30]) and the QOLIBRI (Quality Of Life after traumatic Brain Injury) [31]. The QOLBI consists of 35 items covering six domains: physical, intellectual, psychological, functional, social, and personal. Each item could be rated from 1 (not at all satisfied) to 10 (very satisfied). Psychometric analysis of the QOLBI led to the development of the QOLIBRI, which consists of 37 questions probing the individual's satisfaction level in various life domains: (A) thinking and cognition; (B) emotions; (C) autonomy in daily life; (D) social relationships; (E) feelings; and (F) physical condition. Responses are provided on a five-point scale: not at all satisfied, slightly satisfied, moderately satisfied, quite satisfied, very satisfied. We created a questionnaire containing the 16 items common to both versions. Participants could respond on a five-point Likert scale (from 1, not at all satisfied, to 5, very satisfied). Participants could then obtain a score out of 80. A higher score indicated a better quality of life for the participant.
Data Analysis
To describe the long-term evolution of TBI individuals, descriptive statistics were conducted for cognitive, autonomy, and quality of life measures. The 26 participants who had complete scores on the MoCA test were divided into 2 groups based on the time elapsed since the TBI (Table 1). Mean comparisons using non-parametric Mann-Whitney tests were performed to identify differences between the two groups regarding cognitive abilities, social participation, autonomy, and quality of life.
Finally, in an attempt to identify factors that might influence the aging process of TBI individuals in terms of their social participation, the small sample size did not allow for the use of inferential statistical methods. However, we conducted correlations using Spearman's rho coefficient between social participation and cognitive impairments, autonomy level, and quality of life for both groups based on the time elapsed since the TBI.
The statistical analyses were conducted using JAMOVI software version 2.2 (2021).
Results
Evolution of Cognitive Impairments
Subjective Assessment by Participants: In terms of cognitive aspects, participants were asked about the cognitive difficulties they experienced at T1 and T2. We observed that for working memory, episodic memory, divided attention, vigilance, and planning abilities, the majority of participants reported difficulties both at T1 and T2. For simple attention and mental flexibility, the majority of participants did not report any symptoms at either T1 or T2. Finally, few participants experienced complaints that appeared between T1 and T2 (Graph 1).
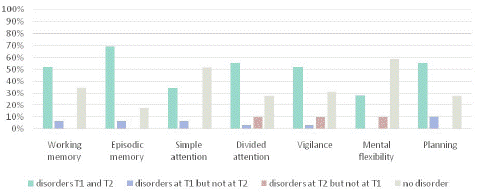
Graph 1: Evolution of cognitive difficulties reported by participants between T1 and T2.
However, when participants were questioned about the evolution of their difficulties between T1 and T2, 17% of participants perceived a significant deterioration in their difficulties, 21% a moderate deterioration, and 7% a minor deterioration. On the contrary, none of the participants reported a significant improvement in their difficulties, 14% reported a minor improvement, 21% reported a moderate improvement, and 7% did not perceive any change. Additionally, 13% were unable to judge their evolution. Therefore, 45% of participants perceived their cognitive performance as deteriorating, while 42% perceived improvement at T2 I (Graph 2).
Graph 2: Evolution of participants’ living arrangements from T0 (pre-TBI) to T2 according to their age at the time of TBI.
Objective Assessment of Global Cognitive Functioning at T2: Participants underwent the MoCA screening test and had an average score of 22.3 out of 30 (min=9; max=29). Only 26 individuals from our sample completed the entire test. Indeed, three individuals were unable to complete the full test due to visual or motor difficulties, or because they chose not to continue. Among these 26 participants, only six (35%) achieved scores within the normal range, which is equal to or higher than 26.
The non-parametric Mann-Whitney test did not show significant differences between the two groups of participants (U=82.5; p=0.959), divided based on the time elapsed since the TBI.
Evolution of Autonomy
The DANEL questionnaire measuring autonomy was used during the data collection interview (T2). The average complex autonomy score was 16.8 out of 46 (SD=11.1, Min=0 – Max=40), and the average basic autonomy score was 3.59 out of 18 (SD=4.48; Min=0 – Max=15). However, in these scores, the responses of "never performs this activity" are not taken into account. Yet, it's possible that TBI individuals never engaged in certain activities because they were not capable of doing so. The DANEL questionnaire allows us to calculate the percentage of autonomy loss while considering the response "never performs the activity." On average, we observed a 35% autonomy loss for complex daily life activities and a 19.9% loss for basic activities.
The non-parametric Mann-Whitney test did not reveal significant differences between the two groups of participants (total autonomy U=80.5; p=0.877; basic autonomy U=83.5; p=1; complex autonomy U=81.5; p=0.918), divided based on the time elapsed since the TBI.
Evolution of Quality of Life
The participants' average score on our questionnaire was 55.4 (SD=11.5; Min=22; Max=75) out of 80. When participants were asked to estimate the evolution of their quality of life between T1 and T2, 83% believed their quality of life had improved at T2, while 14% perceived a deterioration. There appeared to be no difference in terms of age at TBI, age at T2, or the time elapsed since the TBI.
The non-parametric Mann-Whitney test did not indicate significant differences between the two groups of participants (U=81.5; p=0.918), divided based on the time elapsed since the TBI.
Evolution of Social Participation
The social participation of our sample was low in terms of productivity (M=0.88; SD=0.96). The cut-off score for productivity was 0.33, and we observed that 18 participants (62%) scored below the norm, while 11 participants (38%) had social participation equivalent to the healthy population (Table 2). This low score was anticipated, as it corresponds to the subscore that includes work, school, and household participation. Our sample consisted mostly of individuals with severe TBI who had not returned to work. In our sample, 83% of participants were working full-time before their TBI, and 14% were students. Two years after the TBI, 76% were unable to return to work, 24% had resumed some activity, including 7% who were students, 10% had returned to full-time work, 3% to part-time work, and 3% had to take on underqualified jobs. Among the participants who were students at the time of the TBI, 50% were able to resume their studies. At the time of data collection (T2), 17% of our sample had regular jobs, 3% were in a specialized setting for people with disabilities, and 7% were retired at standard retirement age. Among the students, 25% found employment, and 75% were on disability and never started working.
Group
Social participation (PART-O)
Productivity
Social relations
Out and about
Total
5
5-10
25-50
<5
50
10-25
50-75
5-10
<5
10-25
<5
<5
1
<5
<5
<5
<5
1
<5
>75
>75
50-75
1
10
50-75
<5
50-75
1
50
10
50-75
10-20
1
<5
10-25
50-75
<5
1
<5
10-25
25-50
<5
1
10-25
<5
15-25
<5
1
10-25
25-50
<5
<5
1
5
<5
>75
25-50
1
5
>75
50-75
20-25
1
75
= 75
>75
>75
1
5
50-75
50-75
5-10
2
<5
5-10
25-50
<5
2
<5
<5
<5
<5
2
50
25-50
50-75
25-50
2
<5
<5
10-15
<5
2
<5
50
50-75
5-10
2
<5
10-25
25-50
<5
2
<5
50-75
<5
<5
2
10
50
>100
50-75
2
10-25
<5
10-25
<5
2
5
10-25
5-10
<5
2
= 75
= 75
25-50
>75
2
5
10-25
>100
25-50
2
5
5-10
25-50
<5
2
75
25-50
50-75
50-75
Note:The social participation scores correspond to percentiles based on the norms from Holin et al. (in preparation). Bold values represent pathological scores. 1 = Time elapsed since TBI between 10 and 18 years. 2 = Time elapsed since TBI more than 19 years.
Table 2: Distribution of standardized social participation scores assessed with the PART-O scale.
The score for social relations was higher (M=2.4; SD=1.02). Indeed, 79% of the sample fell within the norm, while six participants had deficient scores. For the score of out and about (M=1.11; SD=0.52), 79% were within the norm, and six participants had a deficient score. Lastly, total participation was low for almost half of the participants, with 48% having a social participation score below the norm (Table 2). The non-parametric Mann-Whitney test did not show significant differences between the two groups of participants for any social participation index (productivity U=72.5; p=0.550; social relations U=77.5; p=0.757; out and about U=60.5; p=0.235; total U=71.0; p=0.527), divided based on the time elapsed since the TBI. Finally, regarding protective measures, 45% of our sample were under guardianship, 7% under trusteeship, and 52% had no legal measures in place.
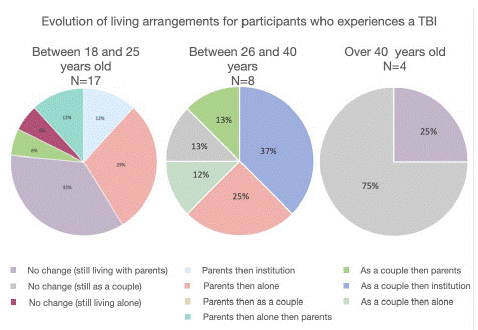
Living space: We could observe a change in living situations between T1 and T2. Before the TBI, all the participants lived with family (55%) or as a couple (55%). Two years after the TBI, 59% of our participants were living with their parents, 24% were in a relationship, 7% were living alone, and 10% were in institutions (MAS or rehabilitation centers). At T2, 28% were living alone, 31% were living with their parents, 24% were in a relationship, and 17% were in institutions (MAS, FAM, shared housing, and therapeutic apartment). The age at which the TBI occurred could explain the living situation before the TBI (Figure 2). Indeed, when the TBI occurred after the age of 40, the majority of participants continued to live in a relationship. For individuals who had a TBI between the ages of 18 and 25, most never left their parents' home. On the other hand, when the TBI occurred between the ages of 26 and 40, it was common for individuals to live with their parents or be in a relationship before the TBI, and then to become independent or separate after the TBI. That's why more of them were living alone at T2 (Graph 2).
Relationship between Social Participation and Other Study Parameters
Spearman's rank correlation coefficients were calculated between the four subscores of social participation (productivity, social relations, out and about, and total score), the three subscores of autonomy (total autonomy, autonomy for basic daily activities, and autonomy for elaborate activities), and quality of life for both groups of participants.
No significant correlations were found for the group of participants who were more than 19 years post-TBI at the time of data collection. However, for the group with a shorter time since TBI (between 10 and 18 years), a significant correlation was observed between total participation and basic autonomy (p=-0.58; p=0.030). Additionally, the scores obtained from the MoCA test were correlated with the "total participation" subscore (p=0.55; p=0.040), total autonomy (p=0.64; p=0.014), elaborate autonomy (p=0.55; p=0.040), and basic autonomy (p=0.58; p=0.030) Participants with higher MoCA scores tended to have better scores in social participation and autonomy.
However, no significant correlation was found between social participation and quality of life at T2.
Discussion
The objectives of this study were to describe the population in the long-term aftermath of TBI to gather information about the aging process of these individuals in terms of the evolution of cognitive abilities, autonomy, quality of life, and social participation at least 10 years after the TBI. The study aimed to investigate whether the time elapsed since the TBI influenced cognitive abilities, autonomy, social participation, and quality of life. The hypothesis was formulated that social participation could serve as a marker for the quality of aging after a TBI. Therefore, the study examined the influence of long-term cognitive deficits, autonomy, and quality of life on social participation based on the time elapsed since the TBI. A comparison was made between cognitive abilities, social participation, autonomy, and quality of individuals who were 10 to 18 years post-TBI at the time of data collection and those who were 19 years or more post-TBI.
No significant differences were found, indicating that the time elapsed since the TBI is not a factor that influences the aging process. This outcome was supported by the similarity between the two participant groups in terms of age, gender, TBI severity, age of TBI onset, and socio-cultural level. Consequently, the lack of significance suggests that the time elapsed does not impact the long-term evolution, indicating that aging in individuals with TBI who are not affected by neurodegenerative conditions is determined by multiple factors.
Evolution of Cognitive Deficits
On the cognitive aspect, the MoCA rapid screening test showed an overall cognitive efficiency that mostly fell below the norms. Furthermore, participants tended to report an increase in cognitive difficulties as they aged. However, it's challenging for individuals to accurately assess their cognitive complaints and their evolution [32]. Since cognitive impairments are at the core of issues faced by individuals with TBI [2,3], they may have focused on their cognitive deficits and tend to magnify them. Additionally, Vallat-Azouvi et al. (2021) [33] found that episodic memory deteriorated eight years after TBI. Given that memory complaints are the most common after TBI [2], individuals who experience these may solely rely on their memory abilities to judge their overall cognitive capabilities, which could also explain the perceived experiences of individuals with TBI.
Evolution of Autonomy
This study also highlights a decline in autonomy among individuals with TBI, particularly noticeable for more complex daily activities compared to basic activities. These findings have been consistent in the literature for some time. For instance, Mazaux et al. [34] previously demonstrated that five years after the trauma, complex daily activities such as handling administrative tasks, financial management, letter writing, calculations, driving, weekly planning, and using public transportation were the most impaired. Our study has revealed similar results at least ten years after the TBI.
Evolution of Quality of Life
Regarding quality of life, it appeared to improve several years after the TBI in our sample. Quality of life is a broad concept that can be influenced by multiple factors such as physical health, psychological state, level of independence, social relationships, and one's relationship with essential elements of their environment [35]. As a result, these results can be explained in various ways. For instance, some of our participants who were students at the time of the TBI were living with their parents and are now independent. Other individuals who experienced the TBI at a later age had older children who could have changed the family dynamic, thus contributing to the decrease in quality of life. Indeed, we observed an evolution in living arrangements that differed based on the age of TBI onset. Those who sustained a TBI at a young age generally lived with their families at the time of the TBI. Later on, they might regain their independence and live alone or continue living with family members, often with a parent serving as a primary caregiver. Similarly, individuals who experienced a TBI at a more advanced age were often living with their families at the time of the TBI and continued to live with their spouse as a primary caregiver. Those who were in a relationship at the time of the TBI were also often found to be living alone several years after the TBI due to the departure of their partner. These findings were consistent with previous studies [36] and are explained by the challenges posed by the TBI, without necessarily establishing a direct link with aging. In other words, beyond the TBI and its functional consequences, the life context can influence the quality of life independently of the TBI. Additionally, a patient's acceptance of their new state can impact their level of quality of life. The TBI, with its resulting sequelae, requires adaptation and the establishment of new plans in social, marital, parental, professional, and recreational aspects. Thus, an individual who persistently aims to return to their professional activity even if they will never be capable of doing so, for instance, may experience distress that could even lead to depression [37]. Unfortunately, depression was not measured in our study due to time constraints.
Evolution of Social Participation
The PART-O questionnaire, which measures social participation, highlighted that over half of the participants had an average total social participation score lower than what is typically observed in the general population, mainly due to the productivity subscore. These findings are not surprising given that a significant number of participants in our sample had not returned to work or education. However, we did observe good scores in social relations and outdoor activities. TBI often impacts social relationships [36], yet it is frequently noted that family bonds are strengthened [38]. Consequently, individuals with TBI can remain well-integrated socially. Moreover, individuals might be part of associations or engaged in specialized medical-social structures for brain-injured individuals, as was the case for 30% of our sample (15% with residential care and 15% in day programs). As a result, they are offered a variety of activities, enabling them to engage out and about and maintain numerous social connections.
Factors that can Influence Social Participation
Social participation, considered a factor of aging well [25] is associated with a reduced risk of disability [39], and is a key element in the study of aging. Furthermore, social participation can influence cognitive abilities and is linked to a lower risk of age-related cognitive decline in healthy individuals [40]. Our study highlights a significant correlation between cognitive abilities and the social participation score. As previously shown in scientific literature, particularly in the context of neurodegenerative diseases, social participation contributes to cognitive reserve, which in our context would help cope with the brain injuries caused by TBI and their long-term consequences [41].
We sought to identify other factors that could be related to social participation. Correlational statistics revealed a link between social participation and cognitive abilities for the group of participants who were 10 to 18 years post-TBI at the time of data collection. However, we cannot conclude that social participation limits cognitive decline, as it could be the case that individuals with better cognitive abilities have greater social participation. Additionally, we did not find these results for the second group of participants. Given our limited sample size, further studies with larger samples are needed to determine whether social participation can indeed mitigate cognitive decline and consequently reduce the loss of autonomy associated with aging.
Limits
This study has several limitations. The number of participants is relatively small considering the inclusion criteria and the challenges of recontacting individuals who are no longer in a medical pathway. The small sample size restricted the number of factors that could be included to explain the aging of individuals with TBI. Furthermore, the size and heterogeneity of the sample limited the statistical power of the study. On the cognitive aspect, we interviewed patients about their evolution between two years post-TBI and at least ten years after TBI; there might be a memory bias that could distort some of the results. Individuals with TBI often have anosognosia [2]. However, to mitigate this bias, we also interviewed caregivers who are with the TBI individuals on a daily basis, especially to assess their autonomy.
Despite these limitations, this study, which includes individuals who suffered from medically confirmed moderate to severe TBI, adds to the limited research focused on the aging of individuals with TBI. It describes the TBI population at least ten years after their injury in terms of cognition, autonomy, social participation, and quality of life.
Conclusion
This study highlights that being a victim of moderate to severe TBI does not systematically lead to long-term cognitive decline. The evolution of cognitive abilities appears to be highly heterogeneous. The time elapsed since the TBI does not seem to influence the aging process in TBI individuals. Thus, this study’s results suggest that TBI individuals’ aging might be characterized by a premature decline in cognitive capacities due to the TBI, but not necessarily an accelerated aging process [5]. Our findings align with those of Hicks et al. (2021) [24].
Furthermore, following this study, we observed that reaching out to TBI individuals several years after their injury has been beneficial for some who were no longer receiving care and were facing aging-related challenges. Many patients complained about the lack of long-term follow-up after a TBI. Therefore, this study has helped identify the needs of patients and their families, aiming to improve their care. Additionally, this study suggests that social participation could be associated with better aging outcomes after a TBI, as is the case in the general population. As a result, promoting social participation is essential to increase the likelihood of healthy aging.
In conclusion, this study provides valuable insights into the long-term consequences of TBI and challenges the assumption of uniform cognitive decline among TBI individuals. It highlights the importance of continued care and support for TBI survivors as they age and underscores the potential benefits of social engagement in promoting healthy aging outcomes.
Author Statements
Acknowledgments
We thank the International Foundation for Applied Research on Disability (FIRAH) and the Haut-de-France Regional Council for funding Samantha Holin's PhD fellowship as part of the present study. We also thank all the participants who participated in the study.
References
- Bayen é, Jourdan C, Azouvi P, Weiss J-J, Pradat-Diehl P. Prise en charge après lésion cérébrale acquise de type traumatisme crânien. Inf Psychiatr. 2012; 88: 331-7.
- Azouvi P. Les troubles cognitifs des traumatismes crâniens sévères. Lett Med Phys Readapt. 2009; 25: 66-8.
- Vallat-Azouvi C, Chardin-Lafont M. Les troubles neuropsychologiques des traumatisés crâniens sévères. Inf Psychiatr. 2012; 88: 365.
- Christensen BK, Colella B, Inness E, Hebert D, Monette G, Bayley M, et al. Recovery of cognitive function after traumatic brain injury: A multilevel modeling analysis of Canadian outcomes. Arch Phys Med Rehabil. 2008; 89: S3-15.
- Wood RLl. Accelerated cognitive aging following severe traumatic brain injury: a review. Brain Inj. 2017; 31: 1270-8.
- Chu BC, Millis S, Arango-Lasprilla JC, Hanks R, Novack T, Hart T. Measuring recovery in new learning and memory following traumatic brain injury: A mixed-effects modeling approach. J Clin Exp Neuropsychol. 2007; 29: 617-25.
- Fraser EE, Downing MG, Biernacki K, McKenzie DP, Ponsford JL. Cognitive reserve and age predict cognitive recovery after mild to severe traumatic brain injury. J Neurotrauma. 2019; 36: 2753-61.
- Millis SR, Rosenthal M, Novack TA, Sherer M, Nick TG, Kreutzer JS, et al. Long-term neuropsychological outcome after traumatic brain injury. J Head Trauma Rehabil. 2001; 16: 343-55.
- Till C, Colella B, Verwegen J, Green RE. Postrecovery cognitive decline in adults with traumatic brain injury. Arch Phys Med Rehabil. 2008; 89: S25-34.
- Mollayeva T, Mollayeva S, Pacheco N, D’Souza A, Colantonio A. The course and prognostic factors of cognitive outcomes after traumatic brain injury: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2019; 99: 198-250.
- Le Gall C, Lamothe G, Mazaux JM, Muller F, Debelleix X, Richer E, et al. Outcome of the Aquitaine Unit for Evaluation, Training and Social and Vocational Counselling (UEROS) at 5-year follow-up in young adults with brain damage. Ann Readapt Med Phys. 2007; 50: 5-13.
- Reuter-Lorenz PA, Cappell KA. Neurocognitive aging and the compensation hypothesis. Curr Dir Psychol Sci. 2008; 17: 177-82.
- Ska B, Joanette Y. Vieillissement normal et cognition. ms. Med Sci (Paris). 2006; 22: 284-7.
- brunel M, Carrère A, Direction de la Recherche, des études, de l’évaluation et des Statistiques. Incapacités et perte d’autonomie des personnes âgées en France: une évolutionfavorable entre 2007 et 2014 – premiers résultats de l’enquête Vie quotidienne et santé 2014. Paris: ministère des Solidarités et de la Santé; n.d.
- Frémont P. Aspects cliniques de la dépression du sujet âgé. Psychol Neuropsychiatr Vieillissement. 2004; 2: 19-28.
- Himanen L, Portin R, Isoniemi H, Helenius H, Kurki T, Tenovuo O. Longitudinal cognitive changes in traumatic brain injury: A 30-year follow-up study. Neurology. 2006; 66: 187-92.
- Wood RL, Rutterford NA. Long-term effect of head trauma on intellectual abilities: a 16-year outcome study. J Neurol Neurosurg Psychiatry. 2006; 77: 1180-4.
- Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: the evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003; 74: 857-62.
- Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM risk factors research group. Int J Epidemiol. 1991; 20: S28-35.
- Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001; 24: 386-91.
- Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, et al. Head injury and the risk of AD in the Mirage study. Neurology. 2000; 54: 1316-23.
- Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 2014; 71: 1490-7.
- Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000; 55: 1158-66.
- Hicks AJ, Spitz G, Rowe CC, Roberts CM, McKenzie DP, Ponsford JL. Does cognitive decline occur decades after moderate to severe traumatic brain injury? A prospective controlled study. Neuropsychol Rehabil. 2022; 32: 1530-1549.
- Gangbè M, Ducharme F. Le ”bien vieillir”: concepts et modèles. Med Sci (Paris). 2006; 22: 297-300.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005; 53: 695-9.
- Delecroix H. Autonomie dans les actes élaborés de la vie quotidienne de la personne cérébro-lésée: quelle évaluation? (Bilan neuropsychologique VS nouveau questionnaire d’autonomie) – Annales de Médecine Physique et de Réadaptation – 52S – e36 – e38 – N° 99. n.d.
- Bogner JA, Whiteneck GG, Corrigan JD, Lai JS, Dijkers MP, Heinemann AW. Comparison of scoring methods for the participation assessment with recombined tools–objective. Arch Phys Med Rehabil. 2011; 92: 552-63.
- Whiteneck GG, Dijkers MP, Heinemann AW, Bogner JA, Bushnik T, Cicerone KD, et al. Development of the participation assessment with recombined tools–objective for use after traumatic brain injury. Arch Phys Med Rehabil. 2011; 92: 542-51.
- Tazopoulou E. Generic and specific measures of quality of life after traumatic brain injury: initial validation of a new specific measure, the QOLBI. Acta Neuropsychol. n.d.;3(1/2):13-24.
- Truelle JL, Koskinen S, Hawthorne G, Sarajuuri J, Formisano R, Von Wild K, et al. Quality of life after traumatic brain injury: the clinical use of the QOLIBRI, a novel disease-specific instrument. Brain Inj. 2010; 24: 1272-91.
- McKinlay WW, Brooks DN. Methodological problems in assessing psychosocial recovery following severe head injury. J Clin Neuropsychol. 1984; 6:87-99.
- Vallat-Azouvi C, Swaenepoël M, Ruet A, Bayen E, Ghout I, Nelson G, et al. Relationships between neuropsychological impairments and functional outcome eight years after severe traumatic brain injury: results from the PariS-TBI study. Brain Inj. 2021; 35: 1001-10.
- Mazaux JM, Masson F, Levin HS, Alaoui P, Maurette P, Barat M. Long-term neuropsychological outcome and loss of social autonomy after traumatic brain injury. Arch Phys Med Rehabil. 1997; 78: 1316-20.
- What quality of life? The WHOQOL group. World Health Organization quality of life assessment. World Health Forum. 1996; 17: 354-6.
- Webster G, Daisley A, King N. Relationship and family breakdown following acquired brain injury: the role of the rehabilitation team. Brain Inj. 1999; 13: 593-603.
- Seel RT, Kreutzer JS. Depression assessment after traumatic brain injury: an empirically based classification method11No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated. Arch Phys Med Rehabil. 2003; 84: 1621-8.
- Dumont C, Gervais M, Fougeyrollas P, Bertrand R. Toward an explanatory model of social participation for adults with traumatic brain injury. J Head Trauma Rehabil. 2004; 19: 431-44.
- Lund R, Nilsson CJ, Avlund K. Can the higher risk of disability onset among older people who live alone be alleviated by strong social relations? A longitudinal study of non-disabled men and women. Age Ageing. 2010; 39: 319-26.
- Tomioka K, Kurumatani N, Hosoi H. Social participation and cognitive decline among community-dwelling older adults: A community-based longitudinal study. J Gerontol B Psychol Sci Soc Sci. 2018; 73: 799-806.
- Stern Y. The concept of cognitive reserve: A catalyst for research. J Clin Exp Neuropsychol. 2003; 25: 589-93.