Abstract
Since upper endoscopy is widely available, clinicians more often encounter the bulges arising beneath the epithelium, with variable clinical significance ranging from insignificant to malignant lesions. When endoscopic biopsies obtained from mucosa cannot determine the diagnosis Endoscopic Ultrasound (EUS) is recommended to ascertain the size, layer of origin, features of echogenicity, and high-risk features for malignancy suspicion to define the probable diagnosis. Current review outlines the EUS features of gastric subepithelial lesions with special focus on small hypoechoic solid lesions originating from muscularis propria which commonly turn out to be gastrointestinal stromal tumors.
Keywords: Subepithelial lesions; Muscularis propria; Endoscopic ultrasound (EUS); Echogenicity
Introduction
Since upper gastrointestinal system endoscopy is widely available, clinicians more often encounter the bulges arising beneath the epithelium, with variable clinical significance ranging from insignificant to malignant lesions. Endoscopic biopsies can unlikely determine the diagnosis because these lesions usually lie deep in the GI wall. For the Subepithelial Lesions (SELs) larger than 10 mm, evaluation with EUS is recommended to ascertain the size, layer of origin, features of echogenicity, and high-risk features for malignancy suspicion [1].
Because small SELs of stomach (<2 cm in diameter) are difficult to detect by other non-invasive radiological methods such as Transabdominal Ultrasound (US), Computerized Tomography (CT) or Magnetic Resonance Imaging (MRI), EUS is the major tool guiding the clinician towards the possible diagnosis.
The consecutive hyper- and hypo-echoic layers of the gastrointestinal tract observed by conventional EUS correspond to the histological wall layers. Considering the gastric wall from lumen towards the serosa, layers of submucosa (3rd EUS layer) and muscularis propria (4th EUS layer) are of major concern since the potentially malignant lesions located in that area and standard endoscopes cannot evaluate those deep structures. Additionally, the echogenic composition of the lesion can give us some clues about the nature of it. Based on the echogenic feature of the wall lesion, hyperechoic lesions are usually benign, most common of which are lipomas. Anechoic lesions located in the submucosa are cystic lesions such as duplication cysts or subepithelial varices. Hypoechoic, heterogeneous lesions usually located in the submucosa of the gastric antrum, having duct like interior spaces may suggest aberrant pancreas and usually poses typical umblicated mass lesion on endoscopic view. The small hypoechoic solid lesions (<2 cm in diameter) with well-defined margins originating from the muscularis propria of the gastric wall suggest Gastrointestinal Stromal Tumors (GISTs) and exhibit many dilemmas for the clinician. Those can be summarized as difficulties in excluding causes other than GISTs (such as leiomyomas, aberrant pancreas, schwannomas and neuroendocrine tumors) (Table 1), poor diagnostic yield after EUS guided fine needle aspiration (EUS-FNA), ambiguity in predicting the malignant potential of disease in GISTs [2].
Diagnosis
Layer of origin
Echogenic appearance
Carsinoid Tumor
Second /Third
Mostly hypoechoic, less common isoechoic
Inflammatory fibroid polyp
Second /Third
Hypoechoic
Granular cell tumor
Second /Third
Hypoechoic
Aberrant pancreas
Third /May expand to second and third
Hypoechoic
Lipoma
Third
Hyperechoic
Varix
Third
Anechoic
Schwannoma
Forth
Hypoechoic
Leiomyoma
Forth / Rarely second
Hypoechoic
Gastrointestinal stromal tumor
Forth / Rarely second
Hypoechoic
Table 1: EUS Features of the Gastric Subepithelial Lesions regarding the layer of origin and echogenic appearance.
Evaluation of the Small Gastric Subepithelial Lesions
In most cases, EUS findings only allow a presumptive diagnosis and determine the need for further explorations such as tissue sampling, surgery, or follow-up. Hypoechoic lesions originating from the muscularis propria (fourth layer) of the viscous wall are mostly GISTs (Figure 1) if they are localized in the stomach, but EUS alone is not sufficient to differentiate GIST from other causes of hypoechoic muscularis propria masses like schwannoma, leiomyoma, lymphoma, etc. therefore, a tissue diagnosis with EUS-FNA is generally performed.
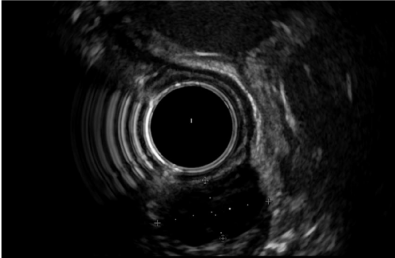
Figure 1: An incidental small hypoechoic lesion (calipers) from muscularispropriain gastric wall (15.3×8.2 mm). Endoscopic ultrasound assisted fine needle
aspiration was performed due to the presence of echogenic foci and the result was gastrointestinal stromal tumor.
The diagnostic yield of EUS-FNA for small gastric hypoechoic SELs was reported as 73% (66/90 patients) in a recent study [3]. Histological diagnosis of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) showed 44 GISTs, 1 glomus tumor, 1 SEL like cancer, 1 malignant lymphoma, 14 leiomyomas, 4 ectopic pancreas, and 1 neurinoma. Of those lesions leiomyoma, ectopic pancreas, and neurinoma were benign lesions that do not warrant further treatment [3]. Among those patients, 44 were surgically resected and the diagnostic accuracy of EUS-FNA with reference to post-operative pathology results was 98% (43/44). Thus, GIST is the most common etiology of hypoechoic gastric SELs and EUS-FNA may count an accurate and safe method in the diagnosis of gastric SELs smaller than 2 cm.
The EUS features of the gastric GISTs for prediction of the malignant potential were investigated. According to the study by Chen et al., tumor size greater than 5 cm, was the only independent risk factor for malignancy (intratumoral calcification and cysts, surface ulceration, patient’s age) [4]. Nevertheless, when the GISTs of between 2 to 5 cm sizes were considered, the risk stratification based on the size of tumor could not be accomplished [5].
At present, although EUS-FNA is considered the procedure of choice for preoperative diagnosis of GIST by immunohistochemical analysis of the sample for c-KIT [6], it provides inadequate material in up to 33.3% of the cases. Particularly, smaller tumors are technically more difficult to obtain adequate histological samples compared to larger ones [7]. So, the diagnosis of small GISTs may be only presumptive based on the EUS appearance but also performing EUSFNA and management decision may be challenging. Nevertheless, GIST appeared to be the most common diagnosis among the MUSCULARIS PROPRIA-SELs in many studies evaluating the post-operative results. In one study, post-operative histological examination revealed GIST in 16 of 19 (84.2%) resected gastric MUSCULARIS PROPRIA-SELs =3 cm in size (the others were 2 schwannomas and 1 leiomyoma) [8]. Even in the case of a GIST diagnosis accomplished by EUS-FNA, evaluation of the malignant potential of the tumor based on the mitotic index may not be possible due to the lack of as sufficient material as required for prompt investigation. Thus EUS-FNA may not change the management strategy at least in a significant subset of patients with asymptomatic small MUSCULARIS PROPRIA-SELs.
The National Institutes of Health (NIH) consensus [9] classified GISTs into very low-, low-, intermediate-, and high-risk categories by using the size and mitotic count of the lesions. Furthermore, Miettinen and Lasota [10] indicated that GISTs =20 mm with a mitotic index of =5/50 HPF have no metastasis risk, thus they defined these lesions as benign, although NIH has avoided such a category. Nevertheless, obtaining sufficient material from a small SEL to scrutinize its malignant potential by assessing the mitotic count may be technically difficult. In such cases, the indirect features suggesting malignant potential during EUS examination may give an idea and help to individualize the treatment especially for those who are not fit for surgery. Those features are large size (=2cm), irregular borders, heterogeneous echotexture, presence of anechoic (cystic) spaces, echogenic foci, and growth during follow-up. Contrast-enhanced endoscopic ultrasound which is not available widely outside of Europe may also give clues suggesting malignant potential that are irregular vascular patterns on vessel images, heterogeneous enhancement due to avascular areas (necrosis) on perfusion images [8].
How to Follow Up the Small Muscularis Propria Subepithelial Lesions
The frequency of follow-up duration of small SELs suggestive of GIST is controversial in different guidelines. The American Gastroenterological Association (AGA) recommends surveillance for <30 mm lesions without concerning the endosonographic features [11]. The US National Comprehensive Cancer Network (NCCN) avoids making a clear statement for the small gastric GISTs (<20 mm) because of the insufficient data, but recommends resection of the small lesions with high-risk EUS features, and endoscopic surveillance at 6- to 12-month intervals for the lesions without high-risk features [12]. European Society for Medical Oncology (ESMO) guidelines suggest a short-term first control (e.g. at 3 months) continuing with a longer interval follow-up schedule in case of no growth, if follow-up strategy is chosen for small lesions [13]. Japanese guidelines recommend that lesions <20 mm in size and without ulceration or surface depression can be managed with endoscopic follow-up once or twice a year [14]. There are no large-scale investigations providing evidence for the effectiveness of these follow-up schedules. The study published by our group has shown that small hypoechoic SELs of <2 cm originating from muscularis propriain stomach suggestive of GIST lesions with EUS survive disease free for a mean duration of 4 years although most patients in our series had much longer surveillance [15]. Therefore, we support the notion of conservative management of small hypoechoic SELs originating from muscularis propria of gastric wall and we summarize it on Figure 2. Our results suggest that following up of those small asymptomatic muscularis propria- SELs detected incidentally during upper gastrointestinal endoscopy without any high risk echogenic features for malignancy not less than 4 years is sufficient. We believe, the guidelines regarding the followup recommendations of small hypoechoic muscularis propria SELs of stomach suggestive of GISTs should be revised as they currently recommend EUS follow-up every 3 to 12 months after the initial examination [16].
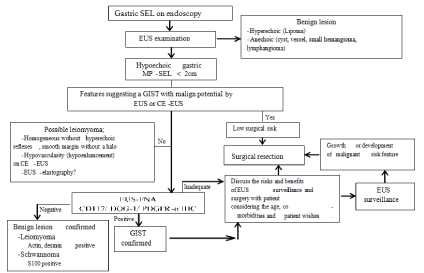
Figure 2: Suggested approach for the management of small gastric MUSCULARIS PROPRIA-SELs.
CE-EUS: Contrast-Enhanced EUS; DOG-1: Discovered on GIST-1; EUS: Endoscopic Ultrasonography; GIST: Gastrointestinal Stromal Tumor; IHC:
Immunohistochemistry; MP: Muscularispropria; PDGFR-a: Platelet Derived Growth Factor Receptor Alpha; SEL: Subepithelial Lesion [16].
Certainly, non-invasive tools such as transabdominal ultrasound, CT or MRI can be used for following the small gastric SELs when the patient is assessed to not to require any surgical or endoscopic resection. In fact, aiming to find an alternative to EUS for follow up of the small gastric SELs, the diagnostic ability of the Multi-Detector CT (MDCT) has been investigated in detecting the gastric SELs <5cm in comparison to Endoscopic Ultrasonography (EUS) as a standard reference [17]. Sensitivity and specificity of MDCT with stomach protocol were 69.6% and 100.0%, respectively. Positive Predictive Value (PPV) and Negative Predictive Value (NPV) of CT were 100.0% and 45.2%, respectively [17]. The cut-off value for prediction of CT visibility was 10 mm and hence, CT using a special protocol may be an alternative to EUS though it exposes the patient to radiation hazards [17]. Nevertheless, as stated above, NCCN and Japanese guidelines also suggest follow up using conventional endoscope for the small gastric SELs which do not exhibit any worrisome EUS feature of malignant behaviour suspicion in the initial evaluation [12,14].
Conclusion
In conclusion drawing definite lines for the management of small gastric GISTs is hindered by the insufficient sample size of studies, since the GISTs are rare tumors. Available data support the notion of a conservative management strategy, rather than a surgical approach, for small gastric muscularis propria-SELs, but in the light of the uncertainties, final decision for these lesions must be individualized after options are thoroughly discussed with the patient. More accurate non-invasive characterization with the newer imaging techniques that allow a better targeted puncture, improvements in design of needles providing larger and safer tissue acquisition, and identification of the molecular and genetic aspects of malignant transformation profiles at the earlier stages of the small gastric muscularis propria-SELs may provide superior risk estimation and refinement of the management strategies in the future. Currently, EUS and EUS-FNA serve an important aid for characterization of all SELs as to whether they are located in or outside of the gastric wall and brief prediction of nature of the lesion.
References
- Hwang JH, Kimmey MB. The Incidental Upper Gastrointestinal Subepithelial Mass. Gastroenterology. 2004; 126: 301-307.
- Papanikolaou IS, Triantafyllou K, Kourikou A, Rösch T. Endoscopic ultrasonography for gastric submucosal lesions. World J Gastrointest Endosc. 2011; 3: 86-94.
- Akahoshi K, Oya M, Koga T, Koga H, Motomura Y, Kubokawa M, et al. Clinical usefulness of endoscopic ultrasound-guided fine needle aspiration for gastric subepithelial lesions smaller than 2 cm. J Gastrointestin Liver Dis. 2014; 23: 405-412.
- Chen TH, Hsu CM, Chu YY, Wu CH, Chen TC, Hsu JT, et al. Association of endoscopic ultrasonographic parameters and gastrointestinal stromal tumors (GISTs): can endoscopic ultrasonography be used to screen gastric GISTs for potential malignancy? Scand J Gastroenterol. 2016; 51: 374-377.
- Kim MN, Kang S, Kim S, Im JP, Kim JS, Jung HC, et al. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver. 2013; 7: 642-647.
- Akahoshi K, Oya M. Gastrointestinal stromal tumor of the stomach: How to manage? World J Gastrointest Endosc. 2010; 2: 271-277.
- Akahoshi K, Sumida Y, Matsui N, Oya M, Akinaga R, Kubokawa M, et al. Preoperative diagnosis of gastrointestinal stromal tumor by endoscopic ultrasound-guided fine needle aspiration. World J Gastroenterol. 2007; 13: 2077-2082.
- Kim MY, Jung HY, Choi KD, Song HJ, Lee JH, Kim do H, et al. Natural history of asymptomatic small gastric subepithelial tumors. J Clin Gastroenterol. 2011; 45: 330-336.
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002; 33: 459-465.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23: 70-83.
- Hwang JH, Rulyak SD, Kimmey MB. American Gastroenterological Association Institute technical review on the management of gastric subepithelial masses. Gastroenterology. 2006; 130: 2217-2228.
- Demetri GD, von Mehren M, Antonescu CR, DeMatteo RP, Ganjoo KN, Maki RG, et al. NCCN Task Force Report: Update on the Management of Patients with Gastrointestinal Stromal Tumors. JNCCN. 2010; 8: S1-S40.
- The ESMO / European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2012; 23: vii49-vii55.
- Nishida T, Hirota S, Yanagisawa A, Sugino Y, Minami M, Yamamura Y, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: English version. Int J Clin Oncol. 2008; 13: 416-430.
- Yegin EG, Kani T, Banzragch M, Kalayci C, Bicakci E, Duman DG. Survival in patients with hypoechoic muscularis propria lesions suggestive of gastrointestinal stromal tumors in gastric wall. Acta Gastroenterol Belg. 2015; 78: 12-17.
- Yegin EG, Duman D. Small EUS-suspected gastrointestinal stromal tumors of the stomach: an overview for the current state of management. Endosc Ultrasound. 2016; 5: 69-77.
- Ra JC, Lee ES, Lee JB, Kim JG, Kim BJ, Park HJ, et al. Diagnostic performance of stomach CT compared with endoscopic ultrasonography in diagnosing gastric subepithelial tumors. Abdom Radiol (NY). 2016.
