Abstract
Introduction: Methane gas production by gut microbiota has been associated with Chronic Constipation (CC) and delayed intestinal transit. Therapeutic fibre is a substrate for this fermentation and its effects are influenced by gas production. Our trial focuses on the possible deleterious effect of methane production in CC patients treated with fermentable fibre.
Methods: A cross-over, double blind, randomized trial comparing two types of fibre, with different fermentability, partially (Isphagula husk) or poor (methylcellulose). Before and at the end of each 4-week treatment phase we evaluated stool characteristics, colonic transit time and hydrogen/methane production.
Results: Seventy-six patients with CC were evaluable (93% women); mean age was 50.1 years; 19.8% had IBS-C/80.2% functional constipation (Rome III criteria) and 69.7% were methanogenic (M+). Therapeutic response to fibre was similar with Ispaghula and methylcellulose (25.0% vs. 22.4% p=0.62) regardless of methanogenic status. Colonic transit shortened significantly only with Isphagula (Beta=-17.2 h). In M+ group, methylcellulose reduced methane levels (p=0.004) and Isphagula had no effect. Both fibres produced no changes in abdominal pain (p=0.76) and distention (p=0.11).
Conclusions: In chronic constipation neither methanogen status nor fermentative characteristics of fibre influences therapeutic response. Changes in colonic transit may explain different fibre effects in gas production. This randomized trial confirms the results of our previous, not controlled study, which ruled out a deleterious effect of methanogenic status using Ispaghula husk in chronic constipation. Therefore, his easy-to-get biological marker (methanogenic status) is not useful in this context.
Keywords: Functional constipation; Methanogenic flora; Colonic transit time; Ispaghula husk; Methylcellulose
Abbreviations
CC: Chronic Constipation; IBS-C: Constipation-Type Irritable Bowel Syndrome; FC: Functional Constipation; CH4: Methane; H2: Hydrogen; Carbon Dioxide CO2 methanogens M+; Non-Methanogen M–; PPM: Parts Per Million; AUC: Area Under Curve; QoL: Health- Related Quality of Life; BM: Bowel Movements; SBM: Spontaneous Bowel Movement; CSBM: Complete Spontaneous Bowel Movement; Questionnaire of Quality of Life CVE; AE: Adverse Events; SD: Standard Deviation; CI: Confidential Interval; RCTs: Randomized Controlled Trials
Introduction
Functional Chronic Constipation (CC), which comprises Functional Constipation (FC) and constipation-type Irritable Bowel Syndrome (IBS-C), is a prevalent diagnosis in primary care. In this context, fiber has become a cornerstone of initial management [1- 5], and ispaghula husk is the most recommended form [6] because significant improvement in symptoms such as straining, sensation of incomplete evacuation, mean number of stools [7] and accelerated whole-gut transit time by increasing luminal bulk [8,9] has been demonstrated. Ispaghula husk is a soluble fiber highly fermented by microbiota with the production of Methane (CH4), Hydrogen (H2), and other unabsorbed carbohydrates throughout the gastrointestinal tract [10,11]. Hydrogen and methane are excreted in breath, allowing measurement of their production using breath testing [12-17]. Worldwide, a third of healthy individuals without any gastrointestinal symptoms produce methane (M+) [18], and higher prevalences have been found in individuals with different pathological conditions. In a previous study by our research group, we reported an M+ prevalence of 52.6% in healthy individuals and 60.5% in CC patients (p=ns), as well as a significantly higher level of methane production in M+ constipated patients than in M+ healthy controls, with a baseline methane level (ppm) of 22 vs 11; p<0.05 and an AUC (ppm min- 1) of 4350 vs 1679; p<0.05) [19]. Methane is believed to modulate intestinal function [20-23] since Pimentel et al. showed in an animal model that CH4 increases non-propagating small bowel contractile activity and slows intestinal transit [20]. In humans, high levels of methane production are associated with slow intestinal transit and constipation, and methane reduction is associated with constipation improvement [16,19,24-32]. More recently, a microbiota composition analysis showed that Methanobrevibacter smithii is the dominant methanogen during IBS-C. The proportion of Methanobrevibacter smithii in stool correlates well with the amount of methane found in breath [33], and Methanobrevibacter and Akkermansia populations increase with stool firmness and are more prevalent in slow-transit individuals [32].
However, the putative cause/effect relationship among methane, constipation and the possible mechanisms of transit delay are far from clear. Moreover, contrary to methane’s putative deleterious effects, other studies of CC report its positive influence [34]. In a recent study of microbiota during IBS, symptom severity was negatively associated with exhaled CH4 and the presence of methanogens [35]. In fact, some authors claim that methanogenesis may prevent abdominal bloating by reducing the volume of abdominal gas because the synthesis of methane consumes four atoms of hydrogen and one atom of carbon [11,34]. Taking into account these associations, there is increasing interest in methane as an easily accessible biological marker for constipation disorders with the greatest current potential to help identify subgroups of patients responding to specific functional therapies [16,22,23,36].
In this sense, we hypothesized that the presence of methanogenic microbiota influences the host’s response to ingestion of fermentable fiber, which may increase CH4 production and delay colonic transit, causing a worse response to treatment that would not occur during treatment with a nonfermentable fiber. To test this hypothesis, we chose two popular commercially available fiber treatments: ispaghula husk, a highly fermentable fiber and methylcellulose, a nonfermentable; we then tested them in the same CC patients in a crossover randomized controlled trial.
Patients and Methods
Study design
This was a randomized double-blind controlled crossover efficacy study over 12 weeks of two types of therapeutic fiber in CC patients. The study was conducted in three phases (Figure 1): an initial baseline period and two treatment periods with a wash out of two weeks between them. For each treatment period, patients received both an active treatment and a placebo of its counterpart to maintain doubleblind conditions.

Figure 1: Study protocol.
Active treatments were either 3.5 g of ispaghula husk (psyllium) (Plantaben®; Rottafarm SL, Barcelona, Spain) or 250 mg of methylcellulose (Fagron Iberica SAU, Terrassa, Barcelona, Spain) t.i.d. Ispaghula husk and methylcellulose were given at their maximum recommended daily doses according to their product labels. To improve the tolerability of the fiber, it was administered only twice a day during the first week. Ispaghula husk and methylcellulose placebos were prepared to maintain similar appearances and tastes. The ispaghula husk placebo consisted of sachets containing 1.6 g of saccharose (Fragon Iberica), 3 g of the food thickener VEGENATMed ® (Venpharma Laboratorios, Barcelona, Spain), 200 mg of tartaric acid and sodium bicarbonate. The methylcellulose placebo was administered as a capsule of identical presentation, each containing 600 mg of saccharose.
Ispaghula husk sachets, methylcellulose capsules and their corresponding placebos were supplied in kits. Participants were instructed to ingest the contents of one sachet stirred in 200 mL of water and the two capsules before meals three times a day with another glass of water; additionally, participants had to drink sufficient water to achieve approximately 1.5-2 liters of fluid a day.
The study was approved by the Institutional Review Board of Viladecans Hospital and the Ethical Committee of our campus and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments. Clinical trial number is 2010-023958-36 and it is available through the EU Clinical Trials Register (https://eudract.ema.europa.eu/).
Study population
Participants were recruited at the primary care centers in our area and screened at our community hospital. Eligible female and male patients were aged 18-75 years and had idiopathic chronic constipation. At the hospital, the clinical presentation was re-evaluated by the gastroenterologist, and additional investigations were ordered if necessary. The exclusion criteria included patients with alternating constipation and diarrhea or predominant symptoms of obstructive defecation, pregnant or breastfeeding women, history of abdominal surgery (except hysterectomy, cholecystectomy and appendectomy), organic digestive disease, insulin diabetes mellitus, connective tissue disease, or any significant cardiac, neurological, endocrine or oncological disorder. Patients taking opioids, anticholinergics or calcium antagonists were also excluded. Individuals were not eligible if they had started taking psychotropic drugs in the previous month, had used antibiotics or undergone a colonoscopy cleansing procedure in the previous 3 months. Additionally, patients taking fiber supplements, laxatives, or constipating drugs who were unwilling to discontinue these medications for at least 2 weeks prior to the study were excluded. Finally, patients who were unable to follow the study instructions were excluded.
Patients entered the study if a definitive diagnosis of IBS with Constipation (IBS-C) or Functional Constipation (FC) was confirmed following Rome III criteria. IBS classification into the IBS-C subtype was performed based on Bristol Stool Form scale characteristics.
Informed consent was obtained from all individual participants included in the study. After written informed consent was obtained, participants underwent the baseline 2-week period without laxatives. However, rescue laxatives for severe constipation (i.e., at least 3 days after the previous bowel movement) such as oral macrogol, glycerin suppositories or microenemas were allowed during the study, and their use was documented in the diary of daily stools and symptoms. Excluding this change in the use of laxatives, patients were instructed to maintain their usual lifestyle, physical activity and diet throughout all the study periods. After this period, if inclusion criteria were confirmed and exclusion criteria were still absent, the patient was enrolled in the study and was randomized to begin taking ispaghula husk or methylcellulose (Figure 1).
Assessments
Clinical evaluation, compliance to the study protocol and possible adverse events were carried out at inclusion, 6 weeks (on the last day of treatment phase 1) and 12 weeks (on the last day of treatment phase 2). The habitual dietary fiber and water intake was evaluated during the baseline phase.
The following measurements were taken for comparison at the end of the baseline period and at the end of each treatment phase: standardized stool and symptom diary, health-related Quality of Life (QoL), colonic transit time and H2/CH4 breath test.
The participants maintained a standardized diary in which at the end of the day, the patient recorded the number of Bowel Movements (BMs) and the characteristics of each BM, including (1) stool consistency using the 7-point Bristol Scale, (2) presence of straining (yes/no) and (3) sensation of complete bowel emptying (yes/no). A BM was deemed a Spontaneous Bowel Movement (SBM) if no laxative, enema, or suppository was taken in the preceding 4 days or a Complete Spontaneous Bowel Movement (CSBM) if the patient indicated that the SBM was associated with a sensation of complete bowel emptying. Each day, patients also recorded the severity of abdominal discomfort and bloating using a 3-point ordinal severity scale (1_none or mild, 2_moderate, 3_severe).
Each patient’s health-related quality of life was assessed using a specific questionnaire (CVE-20) [37], which evaluates the impact of constipation on 4 domains: emotional, general physical, rectal physical and social quality of life. CVE-20 is the first specific questionnaire in the Spanish language for constipated patients; it is valid, reliable, sensitive to changes and meets the psychometric requirements to be applied in daily practice and clinical trials.
Colonic transit time was assessed by the multiple capsule technique, which requires the ingestion of two capsules containing a total of 20 radio-opaque markers (Marquat Genie Biomedical®, Boissy-Saint-Leger Cedex, France), on days 1, 2 and 3, followed by abdominal X-rays on days 4 and 7 [38-39]. Hydrogen and methane production was measured by a conventional 180 min breath test [40]. All participants underwent breath testing with lactulose (Lainco SA, Rubi, Barcelona, Spain). The day before testing, participants were instructed to consume a carbohydrate-restricted diet to avoid slowdigesting carbohydrates, and there was a 12 h fasting time before the test. Good oral hygiene was recommended. Smoking and exercise were not permitted on the day of testing. Participants were instructed to avoid deep inspiration and not to hyperventilate before exhalation. A fasting H2 level of >10 was considered a dietary indiscretion, and the test was rescheduled. After the breath samples were obtained from the fasting participants, they ingested a solution of lactulose (10 g in 100 mL of water). End-alveolar breath samples were collected at 15-min intervals for 3 h using a 750 mL bag (Quintron Instruments, Milwaukee, WI, USA) and analyzed immediately for the concentrations of H2 and CH4 using a gas chromatography analyzer (Quintron Microlyzer Self-Correcting Model SC; Quintron Instruments). The values were expressed in Parts Per Million (ppm) in room air. A mean methane excretion of ≥2 ppm was used to define participants as “methane producers” (M+). Breath H2 and CH4 measurements for each participant and test were plotted against time in a graph and analyzed using a software package (Prism 3.0 and InStat; GraphPad, San Diego, CA, USA). The following parameters were obtained for breath H2 and CH4: (1) fasting, (2) peak and (3) area under the curve (AUC; ppm/min). We also performed a one-time methane measurement by taking a single breath sample at baseline without an overnight fast or the use of standardized instructions (i.e. inhaling and exhaling normally) through a drinking straw into a test tube (foil bag) for 2-5 seconds.
The primary outcome measure was the complete response to treatment defined as having, in third and fourth weeks of treatment, ≥3 CSBMs per week and an increase of ≥1 CSBM relative to baseline. We also considered a less demanding response, named positive response, when the third and fourth weeks of treatment showed ≥3 SBMs (but not necessarily complete) per week and an increase of ≥1 SBM relative to baseline.
The site investigator assessed all patient-reported Adverse Events (AE) and determined their relationship with study treatment.
Statistical analysis
To estimate the required sample size, we assumed a 60% methanogenic prevalence among our constipated population. Previous studies reported a successful response rate of 50% with fermentable fiber [2]. We considered that a 25% difference in efficacy between treatment groups would be clinically significant. With a crossover design, a sample of 88 patients was required with 80% power, 5% significance and 10% loss to follow-up.
Categorical variables were described using frequencies and percentages. Continuous normal variables were summarized using mean and Standard Deviation (SD). Continuous non-normal variables were summarized using median and interquartile range. Normality was assessed analytically using the Shapiro-Wilk test and graphically using a quantile plot. Mixed-effects models were used to account for the patient clustering and to assess treatment impact on therapeutic response. The residues and conditions of model application were assessed. Group comparisons were reported as baseline-adjusted with 95% confidence intervals. The relationship of one-time methane measure and methanogen status during an LBT was assessed using Fisher’s exact test. Wilcoxon signed-rank sum test was used to examine the changes in continuous non-normal variables. Statistical significance was set at 0.05. Analyses were based on the intention-to-treat principle. Statistical analysis was performed using R version 3.4.3 for Windows.
Results
Primary care physicians invited a total of 323 constipated patients to participate, 271 came to the hospital for the first visit and 135 met all the inclusion criteria. Eventually 88 were randomized as 47 persons declined to participate (Figure 2). Complete paired data were available for the 76 participants who completed both treatment periods and were included in the intention-to-treat analysis.
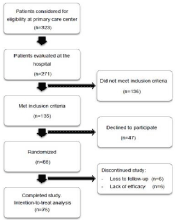
Figure 2: CONSORT diagram showing study enrollment period and participation.
Demographics and baseline characteristics
Baseline characteristics of 76 participants included in the analysis (Table 1). Most of the participants were women, the proportion of FC was 80.2% (n=61), and the remaining patients had IBS-C. These two functional constipation subpopulations had the same proportion of methanogenic cases 42/61 (68.9%) in FC and 11/15 (73.3%) in IBS-C. Furthermore, we observed no differences in baseline mean levels of methane from LBT in the FC patients compared with the IBS-C patients (11.2 [SD 18.5] ppm vs 18.8 [SD 35.7] ppm; p=0.43). In relation to the bowel movements registered by the stool diary the median CSBM was lower in FC compared to IBS-C patients ( 3 [Q1- Q3 2.0-5:0] vs 5 [Q1-Q3 4.0-5:5]); p=0.02). The IBS-C group had higher abdominal pain score than patients with FC (median (SD); 2.0 (0.5) vs 1.5 (0.5); p=0.05). There were no differences in baseline colonic transit time between FC and IBS-C patients (76.8 h [Q1-Q3 40.8.-115] vs 76.8 h [Q1-Q3 58.8-99.6]; p=0.8).
Characteristics
Value
Methane status
methanogenic
53 (69.7%)
non-methanogenic
23 (30.3%)
Sex
Female
71 (93.4%)
Male
5 (6.6%)
Age (years)
Mean (SD)
50.1(13.2)
Median (Q25-Q75)
51.0 (41.0-59.0)
Body mass index
Mean (SD)
25.2 (3.3)
Median (Q25-Q75)
24.9 (22.9-27.1)
Smoking status
never
56 (73.6%)
ex-smoker
3 (4.0%)
smokers
17 (22.4%)
Comorbilities
cardiovascular
16 (21.1%)
digestive
15 (19.7%)
endocrinological
14 (18.4%)
musculoskeletal
12 (15.8%)
respiratory
5 (6.6%)
neurological
9 (11.8%)
depression/anxiety
11 (14.0%)
CVE-20 mean (SD)
11.5 (6.4)
emotional
9.6 (5.1)
general physical
6.4 (3.9)
rectal physical
4.1 (3.0)
social
Time of constipation
>10 years
62 (82.6 %)
5-10 years
6 (8.0 %)
<5 years
7 (9.3 %)
Current laxative use
none
8 (10.5 %)
osmotic agents
35 (46.9 %)
bulking agents
33 (43.8 %)
stimulant laxatives
26 (34.4 %)
Estimated water intake L/day mean (SD)
1.23 (0.43)
Estimated fiber intake g/day mean (SD)
14.8 (5.65)
Table 1: Baseline characteristics of 76 participants in the ITT population.
One-time methane measure
The results of the one-time methane measure show a significant degree of agreement for predicting methanogen status based on the 180 min lactulose breath test (Table 2).
One-time CH4 measure
M- (n=23)
M+ (n=52)
p*
<2 ppm
20 (87%)
5 (9.62%)
<0.001
=2 ppm
3 (13%)
47 (90.4%)
<0.001
LBT: Lactulose Breath Test; CH4: Methane Gas; ppm: Parts Per Million; M-: non-methanogen; M+: Methanogen. Fisher’s exact test.
Table 2: One-time methane measure and the determination of methanogen status by lactulose breath test.
Primary outcome measures
The rate of complete response was 25.0% with ispaghula husk and 22.4% with methylcellulose (p=0.62), although a positive response was higher with both fibers (Figure 3), again without significant differences between the two fibers (p=0.60). The rate of these positive responses for M+ and M- patients (Figure 3). In fact, the multivariate analysis shows the efficacy of treatment is independent of the type of fiber and the patient’s methanogen status (0.72 95% CI 0.20; 2.61, p=0.62). A “carry-over” effect between treatment and period of administration (0.7395% CI 0.12; 4.37, p=0.73) was not observed. The reported levels of product consumption were >80% in both periods.
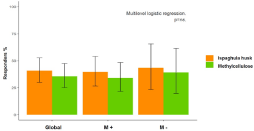
Figure 3: Rate of positive response to laxative therapy in global population
(n=76), methane positive (n=53) and methane negative patients (n=23).
Secondary outcome measures
Abdominal pain and abdominal distention scores at the end of the treatment (Table 3). No differences were found between the treatment groups.
Ispaghula husk
Methylcellulose
p
Abdominal pain score
Median [Q25-Q75]1.50 [1.03; 2.00]
1.57 [1.00; 2.00]
0.76
Abdominal distention score
Median [Q25-Q75]1.93 [1.28; 2.00]
1.93 [1.14; 2.00]
0.11
Table 3: Abdominal symptoms recorded during treatment.
Colonic transit time
A comparison of four-week ispaghula husk consumption vs baseline showed significantly shortened colonic transit times in both M+ and M- patients, while consumption of methylcellulose did not (Figure 4).
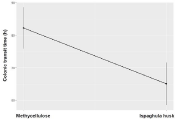
Figure 4: Changes of Colonic Transit Time (CCT) at the end of each
treatment. CCT was singntifically shorter in ispaghula husk group compared
to methylcellulose group (Beta=-17.2 h).
Lactulose breath test analysis
In M+ participants, ispaghula husk treatment did not change gas production during the LBT; with methylcellulose, we observed a significant decrease in breath methane levels in parallel with a significant increase in breath hydrogen levels (Figure 5).
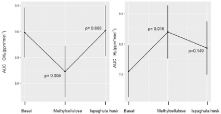
Figure 5: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
In M– patients, neither fibers had an effect on breath methane and hydrogen levels (area under curve, ppm*min-1) (data not shown).
Health-related quality of life
The multivariate analysis demonstrated no significant influence of any of the treatments or methanogen status on the posttreatment scores. In fact, only baseline scores were associated with higher posttreatment scores.
Rescue medication
Eight patients (10.4%) consuming nonfermentable fiber and 3 patients (4.2%) consuming fermentable fiber used rescue medication (p=0.13). In addition, there were no significant differences in rescue medication between methanogen patients (n=6, 5.7%) and nonmethanogen patients (n=5, 11.6%).
Adverse events
Ten patients (13.9%) reported adverse events during methylcellulose treatment, and nine (13.2%) reported adverse events during ispaghula husk treatment. The majority of patients reported abdominal symptoms during the study, such as distension and flatulence (n=5, nonfermentable fiber and n=8, fermentable fiber). No patient reported any serious adverse event. Methanogen status and fiber fermentability did not significantly influence the report of adverse events with respect to treatment (p=0.40 and p=0.93, respectively).
Discussion
Several previously published Randomized Controlled Trials (RCTs) have studied the effects of both fermentable and nonfermentable fiber in individuals with chronic constipation [41- 46], and current guidelines advocate the use of therapeutic fiber as a first line therapy for constipation. The most recent RCT summary presenting the effects of both fermentable and nonfermentable therapeutic fiber in patients with chronic constipation was performed in 2011 by Suares et al., [47], but the majority of these RCTs had a small sample size, and none accounted for baseline dietary fiber consumption. In this regard, we have observed in our study population a poor basal intake of dietary fiber and it is important to say that differences in the amount of dietary fiber intake could influence efficacy results between clinical trials addressing therapeutic fiber in CC.
Our study compares therapeutic doses of methylcellulose and ispaghula husk and shows that both agents exert a modest effect, which is similar to findings of other studies that considered nonrestrictive and more restrictive criteria for responses and included not only stool frequency but also complete evacuation sensation, as pointed out by Dr. Raja and other recently pivotal trials studying the use of laxatives in CC [46,48,49]. In a recent primary care trial, Bijkerk et al., [50] evaluated the efficacy of 10 g of ispaghula (psyllium) over 12 weeks of treatment in a subgroup of patients with C-IBS, showing that this fiber led to improvement in 28% and was more effective than the placebo, which was not significantly different from secondary care findings. Moreover, Hamilton et al., [41] compared various doses of methylcellulose and ispaghula and showed that both agents exerted a modest effect in terms of stool frequency and consistency without differences between agents. Available data suggest that significant modification of colonic motility by ispaghula increases luminal bulk, resulting in increased peristalsis [8]. Currently, there has been increasing research regarding the importance of the gastrointestinal microbiota to gut function and the effect of probiotics on gut motility and constipation. Studies have shown that specific probiotics may help decrease gut transit time in people with or without constipation [51].
Independent of the criteria for responses used, our main goal was to analyze differences in the rates of response between methanogen and non-methanogen patients using a more accurate methodology than in our previous study [19]. We observed that ispaghula husk significantly accelerates transit time without leading to the expected increase in methane production in methanogen patients. This result could be explained by the fact that accelerated intestinal transit time would give less time for gas production during the lactulose breath test. Recent studies report that Methanobrevibacter and Akkermansia are more prevalent in slow-transit individuals, suggesting that changes in colonic transit contribute to colon ecosystem differentiation and act as a strong confounding factor for methanogenic microbiota composition [32,52]. Although H2/CH4 breath testing remains a useful, inexpensive, simple and safe diagnostic tool in gastroenterology, it is well known that it has some limitations, and the interpretation of these tests is subject to discussion; moreover, there is significant heterogeneity in test performance, indications and interpretation of results [16]. Recently, some authors evaluated the relationship between intestinal CH4 production measured in rectal samples and breath excretion of CH4 in a large cohort of IBS patients and have pointed out that breath methane is not an accurate marker of colonic methane, as proposed by DiStefano et al., [53], who suggested breath CH4 excretion should undergo an in-depth revision because this method is not a good marker of CH4 colonic production. Recently, new microbiota quantification techniques permit the introduction of microflora quantification in the equation of gas production. There is controversy as to whether the rate and amount of methane production depend only on the methanogen microflora present in the colon or whether it also depends on gut transit time. Parthasarathy et al., [54] suggest that breath methane production is associated with several genera of Bacteroidetes and Firmicutes that are different from those associated with colonic transit. Moreover, breath methane production was not correlated with colonic transit. They also observed that while the mucosal microbiota is associated with constipation independently of colonic transit, the fecal microbiota is associated with colonic transit and breath methane production. Finally, they suggest that the microbiota profile associated with breath methane is not explained by slow colonic transit.
Wolf et al., [55] evaluated the sigmoid colonic mucosal and fecal abundance of Hydrogenogenic FeFe (FeFe-hydA), hydrogenotrophic Methyl Coenzyme M Reductase A [mrcA], and Dissimilatory Sulfite Reductase A [dsrA]) genes with qPCR assays. They also determined breath hydrogen and methane levels with scintigraphy of 25 constipated females after oral lactulose and colonic transit and observed breath hydrogen and methane were not correlated with constipation, slow colon transit, or with abundance of corresponding genes. Thus, they concluded that breath gases do not directly reflect the abundance of target genes contributing to their production.
One limitation of our study is that we did not analyze the microbiota and therefore cannot correlate microbiota data with changes in gases and colonic transit time. A secondary objective of our work was to correlate changes in abdominal distension or bloating with gas production. In parallel to the absence of an increase in gas production with a fermentable fiber, the participants did not report increased bloating associated with ispaghula husk. Additionally, Levitt et al., [56] evaluated psyllium and methylcellulose in human patients and reported no significant change in the frequency of passed gas and abdominal bloating compared to individuals taking the placebo. Others have also reported no significant increase in rectal expulsion of gas, including CO2, or the excretion of methane and hydrogen in the breath due to psyllium compared to use of a placebo [57,58]. Taken together, our results do not support that gas symptoms reported by some patients as an adverse event have no relationship with amounts of hydrogen and methane gas produced during fermentable fiber treatment. Although they are highly fermented, ispaghula husks do not promote gas generation by gut flora, indicating that mechanisms other than bacterial fermentation could elicit gaseous symptoms during fiber therapy [56,57]. Other authors such as Ghoshal et al., [59] observed that abdominal bloating depends not only on the volume of gas inside the lumen of the gut but also on its preferential retention within the small bowel, as well as on gut motility, visceral sensation and regularity and completeness of defecation [23,59,60]. We have not observed that therapeutic fiber used judiciously, without overdose and with progressive introduction, in a constipated population with a poor basal intake of dietary fiber, can exacerbate problems with abdominal pain or distension. Additionally, no differences were found with respect to quality of life after fiber treatment.
Related to methane status classification, we have demonstrated that a one-time methane measure, not a fasting breath test measure, is sufficient to classify patients as low- and high-methane producers. If CH4 becomes a clinically useful biomarker, this approach can significantly decrease cost and shorten the study time compared to a 180-min lactulose breath test.
Our results are in contrast to those of Pimentel et al., [61], who tested the ability of rifaximin plus neomycin to improve constipation in IBS patients; this improvement depended on the elimination of methane suggesting a pathological role of methanogenesis in these constipated patients.
Our chronically constipated population includes patients with IBS-C and FC; the Rome III criteria define them as mutually exclusive conditions, but there is growing evidence that there is a large overlap between these two functional gastrointestinal disorders. In accordance with this concept, our constipated patients may switch from one category to another depending on the degree of pain over time. The Rome Foundation has made efforts to address these limitations with Rome IV criteria [62].
In conclusion, our study demonstrates that methane does not have a deleterious effect on the response to ispaghula husk treatment in chronic constipation patients. Finally, there is plenty of room for research to identify the mechanism of action of methane on intestinal motility before recommending methane as a biomarker for the diagnosis of constipation-related disorders or as a biomarker for selecting patients who may benefit from specific therapy.
Units
ppm=parts per million, ppp*min-1=parts per million per minute, g=gram, mg=miligram, mL=milliliter
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Funding
This work was supported by FIS grant PI 10/01165 from Instituto de Salud Carlos III (ISCIII) of Spain.
Acknowledgment
We thank Victor Moreno for his methodological support of this work. We also thank Maria Antònia Bernat for her assistance in English revision. We thank CERCA Programme/Generalitat de Catalunya for institutional support. We also thank Lourdes Martos for her effort in conducting the breath tests. We thank Cristian Tebe and Judith Peñafiel for their support in the data analysis.
References
- Bouchoucha M, Faye A, Savarieau B, Arsac M. Effect of an oral bulking agent and a rectal laxative administered alone or in combination for the treatment of constipation. Gastroenterol Clin Biol. 2004; 28: 438-443.
- Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005; 100: 936-971.
- McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998; 12: 491-497.
- Mehmood MH, Aziz N, Ghayur MN, Gilani AH. Pharmacological basis for the medicinal use of psyllium husk (Ispaghula) in constipation and diarrhoea. Dig Dis Sci. 2011; 56: 1460-1471.
- Locke GR 3rd, Pemberton JH, Phillips SF. AGA technical review on constipation. American Gastroenterological Association. Gastroenterology. 2000; 119: 1766-1778.
- Eswaran S, Muir J, Chey W. Fibre and functional gastrointestinal disorders. Am J Gastroenterol. 2013; 108: 718-727.
- Ashraf W, Park F, Lof J, Quigley EM. Effects of psyllium therapy on stool characteristics, colon transit and anorectal function in chronic idiopathic constipation. Aliment Pharmacol Ther. 1995; 9: 639-647.
- Cheskin LJ, Kamal N, Crowell MD, Schuster MM, Whitehead WE. Mechanisms of constipation in older persons and effects of fiber compared with placebo. J Am Geriatr Soc. 1995; 43: 666-669.
- Brownlee IA. The physiological roles of dietary fibre. Food Hydrocoll. 2011; 25: 238-250.
- Levitt MD, Ingelfinger FJ. Hydrogen and methane production in man. Ann NY Acad Sci. 1968; 150: 75-81.
- Gibson GR, Cummings JH, Macfarlane GT. Alternative pathways for hydrogen disposal during fermentation in the human colon. Gut. 1990; 31: 679-683.
- Bond JH, Engel RR, Levitt MD. Factors influencing pulmonary methane excretion in man. J Exp Med. 1971; 133; 572-588.
- Florin TH, Zhu G, Kirk KM, Martin NG. Shared and unique environmental factors determine the ecology of methanogens in humans and rats. Am J Gastroenterol. 2000; 95; 2872-2879.
- Christl SU, Murgatroyd PR, Gibson GR, Cummings JH. Production, metabolism and excretion of hydrogen in the large intestine. Gastroenterology. 1992; 102; 1269-1277.
- Cloarec D, Bornet F, Gouilloud S, Barry JL, Salim B, Galmiche JP. Breath hydrogen response to lactulose in healthy subjects: relationship to methane production status. Gut. 1990; 31; 300-304.
- Rezaie A, Buresi M, Lembo A, Lin H, McCallulm R, Rao S, et al. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am J Gastroenterol. 2017; 112: 775-784.
- Romagnuolo J, Schiller D, Bailey RJ. Using breath test wisely in a gastroenterology practice; an evidence-based review of indications and pitfalls in interpretation. Am J Gastroenterol. 2002; 97: 1113-1126.
- Levitt MD, Furne JK, Kuskowski M, Ruddy J. Stability of human methanogenic flora over 35 years and a review of insights obtained from breath methane measurements. Clin Gastroenterol Hepatol. 2006; 4: 123-129.
- Vega AB, Perello A, Martos L, García-Bayo I, García M, Andreu V, et al. Breath methane in functional constipation: response to treatment with Ispaghula husk. Neurogastroenterol Motil. 2015; 27: 945-953.
- Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Neomycin improves constipation-predominant irritable bowel syndrome in a fashion that is dependent on the presence of methane gas: sub-analysis of a double blind randomized controlled study. Dig Dis Sci. 2006; 51; 1297-1301.
- Low K, Hwang L, Hua J, Zhu A, Morales W, Pimentel M. A combination of rifaximin and neomycin is most effective in treating irritable bowel syndrome with methane on lactulose breath test. J Clin Gastroenterol. 2010; 44; 547- 550.
- Kunkel D, Basseri RJ, Makhani MD, Chong K, Chang C, Pimentel M. Methane on breath testing is associated with constipation: a systematic review and meta-analysis. Dig Dis Sci. 2011; 56; 1612-1618.
- Triantafyllou K, Chang C, Pimentel M. Methanogens, methane and gastrointestinal motility. J Neurogastroenterol Motil. 2014; 20: 31-40.
- Pimentel M, Mayer AG, Park S, Chow EJ, Hasan A, Kong Y. Methane production during lactulose breath test is associated with gastrointestinal disease presentation. Dig Dis Sci. 2003; 48: 86-92.
- Attaluri A, Jackson M, Valestin J, Rao SSC. Methanogenic flora is associated with altered colonic transit but not stool characteristics in constipation without IBS. Am J Gastroenterol. 2010; 105; 1407-1411.
- Lee KM, Paik CN, Chung WC, Yang JM, Choi MG. Breath methane positivity is more common and higher in patients with objectively proven delayed transit constipation. Eur J Gastroenterol Hepatol. 2013; 25; 726-732.
- Pimentel M, Chow EJ, Lin HC. Eradication of small intestinal bacterial overgrowth reduces symptoms of irritable bowel syndrome. Am J Gastroenterol. 2000; 95: 3503-3506.
- Pimentel M, Chow EJ, Lin HC. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: a doubleblind, randomized, placebo-controlled study. Am J Gastroenterol. 2003; 98: 412-419.
- Pimentel M, Lin HC, Enyati P, van den Burg B, Lee HR, Chen J, et al. Methane, a gas produced by enteric bacteria slows intestinal transit and augments small intestinal contractile activity. Am J Physiol Gastrointest Liver Physiol. 2006; 290: 1089-1095.
- Rezaie A, Chang B, Chua KS, Shari K, Lin E, Pimentel M. Accurate identification of excessive methane gas producers by a single fasting measurement of exhaled methane: a large-scale database analysis. Am J Gastroenterol. 2015; 110: S684.
- Jahng J, Jung IS, Choi EJ, Concklin JL, Park H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol Motil. 2012; 24: 185-190.
- Vandeputte D, Falony G, Vieira-Silva S, Tito RY, Joossens M, Raes J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut. 2016; 65: 57-62.
- Kim G, Deepinder F, Morales W, Hwang L, Weitsman S, Chang C, et al. Methanobrevibacter smithii is the predominant methanogen in patients with constipation-predominant IBS and methane on breath. Dig Dis Sci. 2012; 57; 3213-3218.
- Rana SV, Sharma S, Sinha SK, Kaur H, Sikander A, Singh K. Incidence of predominant methanogenic flora in irritable bowel syndrome patients and apparently healthy controls from North India. Dig Dis Sci. 2009; 54: 132-135.
- Tap J, Derrien M, Tornblom H, Brazeilles R, Cools-Potier S, Dore J, et al. Identification of an Intestinal Microbiota Signature Associated With Severity of Irritable Bowel Syndrome. Gastroenterology. 2017; 152: 111-123.
- Hwang L, Low K, Khoshini R, Melmed D, Sahakian A, Makhani M, et al. Evaluating breath methane as a diagnostic test for constipation-predominant IBS. Dig Dis Sci. 2010; 55; 398-403.
- Perona M, Mearin F, Guilera M, Minguez M, Ortiz V, Montoro M, et al. Quality of life specific questionnaire for constipated patients: development and validation of CVE-20. Med Clin (Barc). 2008; 131: 371-377.
- Moreno-Osset E, Ballester J, Minguez M, Mora F, Benages A. Colonic transit time (segmental and total) in healthy subjects and patients with chronic idiopathic constipation. Med Clin (Barc). 1992; 98: 201-206.
- Husni-Hag-Ali R, Gomez Rodriguez BJ, Mendoza Olivares FJ, Garcia Montes JM, Sanchez-Gey S, Herrerías JM, et al. Measuring colonic transit time in chronic idiopathic constipation. Rev Esp Enferm Dig. 2003; 95: 186-190.
- Solomons NW. Evaluation of carbohydrate absorption: the hydrogen breath test in clinical practice. Clin Nutr. 1984; 3: 71-78.
- Hamilton JW, Wagner J, Burdick BB, Bass P. Clinical evaluation of methylcellulose as a bulk laxative. Dig Dis Sci. 1988; 33: 993-998.
- Bianchi M, Capurso L. Effects of guar gum, ispaghula and microcrystalline cellulose on abdominal symptoms, gastric emptying, orocaecal transit time and gas production in healthy volunteers. Dig Liver Dis. 2002; 34: S129-S133.
- Englyst KN, Liu S, Englyst HN. Nutritional characterization and measurement of dietary carbohydrates. Eur J Clin Nutr. 2007; 61: S19-S39.
- Macfarlane S, Macfarlane GT, Cummings JH. Review article: prebiotics in the gastrointestinal tract. Aliment Pharmacol Ther. 2006; 24: 701-714.
- Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ. Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol. 2006; 40: 235- 243.
- Christodoulides S, Dimidi E, Fragkos KC, Farmer AD, Whelan K, Scott M. Systematic review with meta-analysis: effect of fibre supplementation on chronic idiopathic constipation in adults. Aliment Pharmacol Ther. 2016; 44: 103-116.
- Suares NC, Ford AC. Systematic review: the effects of fibre in the management of chronic idiopathic constipation. Aliment Pharmacol Ther. 2011; 33: 895-901.
- Camilleri M, Kerstens R, Rykx A, Vande-plassche L. A placebo-controlled trial of prucalopride for severe chronic constipation. N Engl J Med. 2008; 358: 2344-2354.
- Tack J, Van Outryve M, Beyens M, Kerstens R, Vandeplassche L. Prucalopride (Resolor) in the treatment of severe chronic constipation in patients dissatisfied with laxatives. Gut. 2009; 58: 357-365.
- Bijkerk CJ, de Wit NJ, Muris JW, Whorwell PJ, Knottnerus JA, Hoes AW. Soluble or insoluble fibre in irritable bowel syndrome in primary care? Randomised placebo controlled trial. BMJ. 2009; 339: b3154.
- Dimidi E, Christodoulides S, Scott SM, Whelan K. Mechanisms of Action of Probiotics and the Gastrointestinal Microbiota on Gut Motility and Constipation. Adv Nutr. 2017; 8: 484-494.
- Falony G, Joossens M, Vieira-Silva S, Wang J, Darzi Y, Faust K, et al. Population level analysis of gut microbiome variation. Science. 2016; 352: 560-564.
- Di Stefano M, Mengoli C, Bergonzi M, Klersy C, Pagani E, Miceli E, et al. Breath methane excretion is not an accurate marker of colonic methane production in irritable bowel syndrome. Am J Gastroenterol. 2015; 110: 891- 898.
- Parthasarathy G, Chen J, Chen X, Chia N, O’Connor HM, Wolf PG, et al. Relationship between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology. 2016; 150: 367-379.
- Wolf PG, Parthasarathy G, Chen J, O’Connor H, Chia N, Bharucha A, et al. Assessing the colonic microbiome, hydrogenogenic and hydrogenotrophic genes, transit and breath methane in constipation. Neurogastroenterol Motil. 2017; 29: 1-9.
- Levitt MD, Furne J, Olsson S. The relation of passage of gas and abdominal bloating to colonic gas production. Ann Intern Med. 1996; 124: 422-424.
- Marteau P, Flourie B, Cherbut C, Correze JL, Pellier P, Seylaz J, et al. Digestibility and bulking effect of ispaghula husks in healthy humans. Gut. 1994; 35: 1747-1752.
- Wolever TMS, Wal P, Spadafora P, Robb P. Guar, but not psyllium, increases breath methane and serum acetate concentrations in human subjects. Am J Clin Nutr. 1992; 55: 719-722.
- Ghoshal U, Shukla R, Srivastava D, Ghoshal UC. Irritable Bowel Syndrome, Particularly the Constipation-Predominant Form, Involves an Increase in Methanobrevibacter smithii, Which Is Associated with Higher Methane Production. Gut Liver. 2016; 10: 932-938.
- Agrawal A, Whorwell PJ. Review article: abdominal bloating and distension in functional gastrointestinal disorders: epidemicology and exploration of possible mechanisms. Aliment Pharmacol Ther. 2008; 27: 2-10.
- Pimentel M, Chatterjee S, Chow EJ, Park S, Kong Y. Rifaximin plus neomycin is superior to neomycin alone in improving multiple C-IBS symptoms. This effect is predicted by a reduction in breath methane. Dig Dis Sci. 2014; 59: 1278-1285.
- Drossman DA. Functional Gastrointestinal Disorders: History, Pathophysiology, Clinical Features, and Rome IV. Gastroenterology. 2016; 150: 1262-1279.
