Abstract
Objective: To identify the risk factors of Splanchnic Vein Thrombosis (SVT) in patients with Acute Pancreatitis (AP) using meta-analysis, to provide reference for early clinical intervention.
Methods: The databases were searched to screen the relevant studies on the risk factors of SVT in AP patients, with a search period from the establishment of the database to January 2024. The included literature was statistically analyzed using Review Manager 5.3 software.
Results: A total of 15 studies were included in this report, including 7100 patients with AP, of which 660 patients had SVT. Meta-analysis results revealed high Modified Computed Tomography Severity Index (MCTSI) score with an Odds Ratio (OR) of 2.22 and 95% Confidence Interval (CI) of 1.41 to 3.50 (P < 0.001), high D-dimer with OR of 1.88 and 95%CI of 1.12 to 3.15 (P = 0.02), smoking with OR of 1.21 and 95%CI of 1.07 to 1.36 (P = 0.002), alcoholic etiology with OR of 3.00 and 95%CI of 1.80 to 5.00 (P < 0.001), high hematocrit with OR of 1.07 and 95%CI of 1.03 to 1.11 (P < 0.001) and necrotizing pancreatitis with OR of 6.94 and 95%CI of 3.21 to 15.02 (P < 0.001) were risk factors for SVT in patients with AP.
Conclusion: Alcoholic etiology, smoking, high D-dimer, high MCTSI score, high hematocrit, and necrotizing pancreatitis were risk factors for SVT in AP patients and medical staff should identify patients at high rise of thrombosis using the results of this study to take corresponding preventive measures.
Keywords: Acute pancreatitis; Venous thrombosis; Risk factors; Meta-analysis
Introduction
Acute Pancreatitis (AP) refers to a common acute abdominal disease caused by the abnormal activation of pancreatic enzymes that have a digestive effect on the pancreas itself and surrounding organs. Splanchnic Vein Thrombosis (SVT) refers to thrombosis in the portal, splenic and mesenteric veins. As one of the serious complications of AP, SVT can cause life-threatening conditions such as portal hypertension, intestinal ischemic necrosis, esophageal and gastric variceal bleeding [1]. The incidence of AP-associated SVT ranges from one to 24% [2-6] and the incidence of SVT can be as high as 50% in necrotizing pancreatitis [7]. The splenic vein is most commonly involved in SVT caused by AP, accounting for up to 74% of cases. The SVT may be clinically asymptomatic in the early stages with insidious onset and is often detected incidentally on imaging, so it is critical to actively prevent and minimize the development of SVT in clinical practice. The clinical factors of SVT in AP patients have not been completely identified, so this study was to explore the possible risk factors of SVT in AP patients, with the aim of identifying positive indicators for predicting SVT in AP patients and allowing early intervention and treatment of patients, to improve their quality of life.
Materials and Methods
Retrieval Strategy
Two researchers independently searched literature published in databases, including PubMed, Web of Science, Cochrane Library, Embase, from the time of their establishment until January 2024. MeSH keywords included pancreatitis, venous thrombosis, risk, combined with literature traceability and manual retrieval. The search results were exported to NoteExpress for further evaluation and disagreements were decided by a third researcher.
Literature Inclusion and Exclusion Criteria
Inclusion criteria
1) Literature type: Case-control studies or cohort studies on risk factors of AP-related SVT published anywhere;
2) Study subjects: Patients who met the diagnostic criteria for AP and developed SVT in the case group or the exposed group and patients who did not develop SVT in the control or unexposed group;
3) There were no language restrictions;
4) The literature which could provide the original data of the odds ratio (OR) value and its 95 % confidence interval (CI) derived from the results of multivariate analysis.
Exclusion criteria
1) Animal experiments or case reports, reviews and meta-analysis;
2) Patients with liver cirrhosis and chronic pancreatitis and those complicated by liver cancer or other malignant tumors or other diseases known to affect coagulation function;
3) Studies with poor quality, repeated reports or no available data.
Data Extraction and Quality Evaluation
Two researchers independently extracted information, including author, publication time, study type, country, number of cases in SVT group, number of cases in non-SVT group, risk factors, OR value and 95 % CI. The Newcastle-Ottawa Scale (NOS) was used to evaluate the quality of the included literature and was composed of three main parts, including the selection of study subjects (four stars), inter-group comparability (two stars) and the measurement of exposure factors (three stars). The star system quantification principle was adopted, with a full score of nine stars. Studies with a score of seven or more stars were considered to be high-quality.
Statistical Methods
Data extracted were statistically analyzed using Review Manager 5.3 software. Meta-analysis was performed on possible individual risk factors of SVT in liver cirrhosis with OR value used as the effect index for categorical variables and intervals estimated using 95% CI.
A heterogeneity test was performed for each included report. If heterogeneity test P = 0.1 and I2 = 50%, heterogeneity among all studies was considered to be low and a fixed effect model was applied to combine statistics. If P < 0.1 and I2 > 50%, there was high heterogeneity among studies and a random effect model was used to combine statistics. Sensitivity analysis was carried out by eliminating the included studies one by one.
Results
Literature Retrieval Results
A total of 1776 relevant studies were retrieved and 653 repeated titles were excluded using NoteExpress software. Of the remaining studies, 1075 reviews, case reports and reports not related to the subject were excluded after reading the titles and abstracts, leaving 15 to be included after full text reading. The literature screening process is shown in Figure 1.
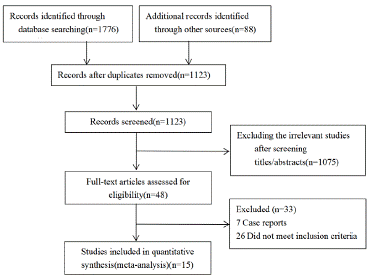
Figure 1: Literature screening process.
Basic Characteristics and Quality Evaluation of the Included Studies
A total of 15 studies were included, including two prospective study and thirteen retrospective study. These contained a total of 7100 patients with AP, including 660 in the SVT group and 6440 in the non-SVT group. With NOS score of more than six points, these all met the quality requirements. Risk factors included MCTSI score, D-Dimer, Prothrombin Time (PT), gender, age, smoking, alcoholic etiology, hematocrit (HCT) and necrotizing pancreatitis. The basic characteristics and quality evaluation results of the included literature are shown in Table 1.
Author
Publication year
Country
Study type
Number of cases(n)
Risk factors included
NOS score
SVT
Non-SVT
Sissingh NJ [8]
2024
Netherlands
Prospective study
97
335
(6)
6
Zheng J [9]
2023
China
Retrospective study
32
145
(3)
7
Yang WX [10]
2023
China
Retrospective study
48
54
(1)(3)
8
Wei ZG [11]
2023
China
Retrospective study
29
113
(1)(2)(4)(9)
8
Yang X [12]
2022
China
Retrospective study
21
192
(1)(3)(4)
8
K T [13]
2022
United Kingdom
Retrospective study
109
292
(2)(8)
8
Huang WQ [14]
2021
China
Retrospective study
29
136
(1)(2)(4)
8
Bjornsson E [15]
2020
Iceland
Retrospective study
20
1082
(2)(8)
6
Ling ZY [16]
2020
China
Retrospective study
27
171
(1)
8
Ding L [17]
2018
China
Retrospective study
25
115
(1)
8
Marra-López C [18]
2017
Spanish
Prospective study
60
1601
(2)(5)(6)
7
Fei Y [19]
2017
China
Retrospective study
35
136
(3)(4)(5)(6)(9)
6
Wang YC [20]
2017
USA
Retrospective study
7
101
(7)
6
Toque L [21]
2015
France
Retrospective study
19
299
(7)(8)
8
Zhou J [22]
2015
China
Retrospective study
42
73
(1)
8
Table 1: The basic characteristics of included reports.
Reference
The number of studies
Heterogeneity test
Effect model
Combined effect amount
I2 (%)
P
OR
95% CI
P
MCTSI score
7
82
<0.01
random
2.22
1.41~3.50
<0.001
necrotizing pancreatitis
5
62
0.03
random
6.94
3.21~15.02
<0.001
D-Dimer
4
89
0.01
random
1.88
1.12~3.15
0.02
PT
3
72
0.03
random
1.07
0.94~1.21
0.33
Gender(male)
3
0
0.57
fixed
1.3
0.95~1.77
0.1
age
3
50
0.14
fixed
0.99
0.99~1.00
0.2
smoking
2
46
0.17
fixed
1.21
1.07~1.36
0.002
alcoholic etiology
3
0
0.54
fixed
3
1.80~5.00
<0.001
HCT
2
0
0.7
fixed
1.07
1.03~1.11
<0.001
Table 2: Summary of meta-analysis results.
Risk factors included: (1) MCTSI score, (2) necrotizing pancreatitis, (3) D-Dimer, (4) Prothrombin Time (PT), (5) gender, (6) age, (7) smoking (8) alcoholic etiology, and (9) hematocrit (HCT).
Meta-Analysis Results
MCTSI score: Seven studies reported the relationship between MCTSI score and SVT in AP patients, with high heterogeneity among studies (P < 0.00001, I2 = 82%). Through sensitivity analysis, it is found that a single document has little influence on the results, and it is impossible to confirm the specific source of heterogeneity in the data. Meta-analysis was performed using a random-effect model and it was found that a high MCTSI score was an independent risk factor for SVT in AP patients with an OR of 2.22 and 95% CI of 1.41 to 3.50 (P < 0.001), as shown in Figure 2.
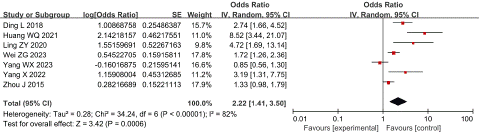
Figure 2: Forest plot of the relationship between MCTSI score and SVT in AP patients.
Necrotizing pancreatitis: Five studies reported the relationship between necrotizing pancreatitis and SVT in AP patients, with high heterogeneity among studies (P = 0.03, I2 = 62%). The sensitivity analysis revealed no significant heterogeneity. The random effect model was adopted for analysis and the results suggested that necrotizing pancreatitis was an independent risk factor for SVT in AP patients, having an OR of 6.94 and 95% CI of 3.21 to 15.02 (P < 0.001), as shown in Figure 3.
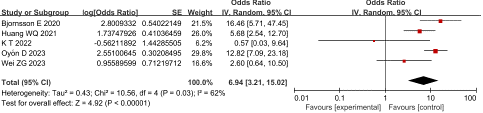
Figure 3: Forest plot of the relationship between necrotizing pancreatitis and SVT in AP patients.
D-Dimer: Four studies reported the relationship between D-Dimer and SVT in AP patients, with high heterogeneity among studies (P=0.01 I2=89%). The sensitivity analysis revealed no significant heterogeneity. The random effect model was adopted for analysis and the results suggested that D-Dimer was an independent risk factor for SVT in AP patients, having an OR of 1.88 and 95 % CI of 1.12 to 3.15 (P = 0.02), as shown in Figure 4.
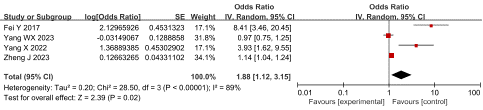
Figure 4: Forest plot of the relationship between D-Dimer and SVT in AP patients.
PT: Three studies reported the relationship between PT and SVT in AP patients, with high heterogeneity among studies (P = 0.03, I2 = 72%). A random-effect model was adopted for analysis and the results showed that PT was not an independent risk factor for SVT in AP patients, having an OR of 1.07 and 95% CI of 0.94 to 1.21 (P = 0.33), as shown in Figure 5.
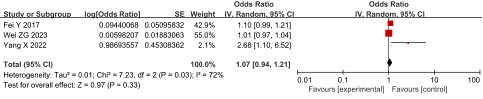
Figure 5: Forest plot of the relationship between PT and SVT in AP patients.
Gender: Three studies reported the relationship between gender and SVT in AP patients, with low heterogeneity among studies (P = 0.57, I2 = 0%). The fixed effect model was adopted for analysis and the results suggested that male was not an independent risk factor for SVT in AP patients, having an OR of 1.30 and 95 % CI of 0.95 to 1.77 (P= 0.10), as shown in Figure 6.
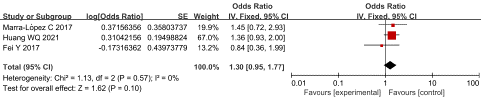
Figure 6: Forest plot of the relationship between gender (male) and SVT in AP patients.
Age: Three studies reported the relationship between age and SVT in AP patients, with low heterogeneity among studies (P = 0.14, I2 = 50%). The fixed effect model was adopted for analysis and the results revealed that age was not an independent risk factor for SVT in AP patients, having an OR of 0.99 and 95 % CI of 0.99 to 1.00 (P= 0.20), as shown in Figure 7.
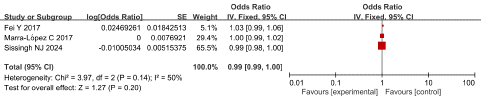
Figure 7: Forest plot of the relationship between age and SVT in AP patients.
Smoking: Two studies reported the relationship between smoking and SVT in AP patients, with low heterogeneity among studies (P = 0.17, I2 = 46%). The fixed effect model was adopted for analysis and the results suggested that smoking was not an independent risk factor for SVT in AP patients, having an OR of 1.21 and 95 % CI of 1.07 to 1.36 (P = 0.002), as shown in Figure 8.

Figure 8: Forest plot of the relationship between smoking and SVT in AP patients.
Alcoholic Etiology: Three studies reported the relationship between alcoholic etiology and SVT in AP patients, with low heterogeneity among studies (P = 0.54, I2 = 0%). The fixed effect model was adopted for analysis and the results showed that alcoholic etiology was an independent risk factor for SVT in AP patients, having an OR of 3.00 and 95 % CI of 1.80 to 5.00 (P < 0.001), as shown in Figure 9.
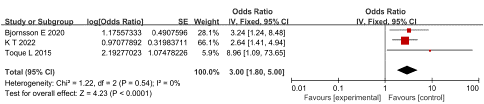
Figure 9: Forest plot of the relationship between alcoholic etiology and SVT in AP patients.
HCT: Two studies reported the relationship between hematocrit and SVT in AP patients, with low heterogeneity among studies (P = 0.70, I2 = 0%). The fixed effect model was adopted for analysis and the results showed that HCT was an independent risk factor for SVT in AP patients, having an OR of 1.07 and 95% CI of 1.03 to 1.11 (P<0.001), as shown in Figure 10.
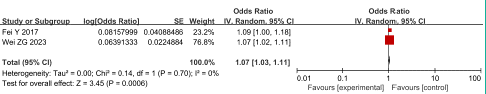
Figure 10: Forest plot of the relationship between HCT and SVT in AP patients.
Discussion
A total of 15 articles were included in this study. The overall incidence of splanchnic vein thrombosis in acute pancreatitis was 9.3%. In recent years, with the popularity of imaging examination, the domestic and foreign literature reported that the detection rate of SVT gradually increased. The risk factors of SVT and the need for anticoagulant therapy have become the focus of experts and scholars. At present, the risk factors of SVT in acute pancreatitis are not completely clear, and the related risks are scattered, including local factors and systemic factors. In this paper, the related studies were summarized and analyzed by the method of meta-analysis, and the risk factors of visceral venous thrombosis in acute pancreatitis were analyzed and discussed.
Systemic Factors
The results of this study revealed that alcoholic etiology and smoking were risk factors, while age and gender were not independent risk factors for SVT in AP patients. In China, cholelithiasis is the main cause of AP, followed by hypertriglyceridemia and excessive drinking [23]. Vascular complications caused by alcoholic pancreatitis are more common than pancreatitis caused by cholelithiasis [24,25]. Chooklin et al. [26] found that alcoholic etiology was a risk factor for portal vein thrombosis in AP patients and it has also been shown [27,28] that patients with alcoholic chronic pancreatitis exhibit a significantly increased risk of vascular complications. In animal models, ethanol increased von Willebrand Factor (vWF) release from the umbilical vein [29] and decreased portal vein motion [30]. A number of studies [31,32] have shown that smoking can significantly increase the risk of SVT in patients with pancreatitis, most likely due to high content of nicotine in the plasma of smokers, which stimulated vasoconstriction, damaged vascular endothelial cells and increased blood viscosity. A large prospective study [33] found that the mechanism of the effect of smoking on venous thrombosis was acute and ex-smokers have essentially the same risk of venous thrombosis as never-smokers. Therefore, clinical staff should advise patients diagnosed with acute pancreatitis to quit smoking immediately to reduce the risk of venous thrombosis. Robbins et al. [34] noted that AP patients who developed venous thromboembolism were older, but this study revealed no statistical significance between age and the occurrence of SVT. A number of studies [14,18,34-36] revealed an increased risk of venous thrombosis in male AP patients, but the results of a comprehensive analysis of three reports [14,18,19] in this study indicated no statistical significance for gender effects in the occurrence of SVT. As only a small number of studies were included in this study, the relationship between age, gender and SVT needs to be further verified. The results of this study also suggested that high D-Dimer and HCT was a risk factor for SVT in patients with AP. These AP patients suffer from vasoconstriction and vascular endothelial cell injury in the portal venous system due to inflammation, fluid loss and endotoxin release, which in turn activates the body coagulation system, resulting in platelet and fibrin-rich thrombin deposition, hypercoagulation and hyperconcentration, inducing venous thrombosis [12]. Fibrin is hydrolyzed into D-Dimer by fibrinolytic enzymes after activation of the fibrinolytic system in humans and it is a common index for detecting venous thrombosis and an early predictor of the severity of AP. This study revealed no statistical significance between PT and the occurrence of SVT. Prothrombin activity exceeding 75% was a protective factor against thrombosis in AP patients [21,37]. Patients with AP have a 3.5-fold and 7.9-fold increased risk of SVT when prothrombin time exceeded 13 seconds and D-Dimer exceeded 4 mg/L, respectively [16]. Changes in coagulation function act at the beginning and end of AP development, which is favorable for assessing thrombosis in AP patients.
Local Factors
Acute pancreatitis with high MCTSI score and necrotizing pancreatitis has an increased risk of developing SVT. MCTSI score included pancreatic inflammatory reaction score and pancreatic necrosis score. Anatomically, the portal vein lies behind the neck of the pancreas and is formed by the confluence of the splenic and superior mesenteric veins. Splenic veins are mostly located behind the body and tail of the pancreas, and pancreatic and peripancreatic edema can compress the portal venous system, slowing down blood flow velocity and blocking splenic venous return. Inflammatory cell infiltration or pancreatic enzyme erosion can directly involve veins and cause vascular intimal injury, resulting in vasospasm, wall thickening, luminal narrowing and ultimately thrombosis. K et al. [13] noted that SVT was closely related to the degree of pancreatic necrosis. The risk of SVT increased by nearly 15 times when the area of pancreatic necrosis was more than 50% and of the 43 patients with SVT, 39 suffered from necrotizing pancreatitis. local Complications of acute pancreatitis is therefore valuable in the early prediction of SVT.
Limitations
There are currently few high-quality studies on the risk factors of SVT in AP patients and the number of studies that can be included in the meta-analysis was also small. As most existing studies were retrospective, it was impossible to comprehensively evaluate the risk factors of SVT in AP patients. Other risk factors such as organ failure, hypoproteinemia, gastrointestinal wall thickening and intra-abdominal pressure had an effect on SVT in AP patients in this study, but meta-analysis was not performed due to only one included study or differing data types. It is therefore recommended that more high-quality, large-sample experimental studies to identify more reliable predictors are carried out.
Conclusion
The results of this study revealed that alcoholic etiology, smoking, high D-dimer, high MCTSI score, high hematocrit, and necrotizing pancreatitis were risk factors for SVT in AP patients and age, gender and PT were not independent risk factors for SVT in AP patients. Medical staff should actively identify patients at high risk of thrombosis using the results of this study, to provide early warning for patients in clinical practice. At present, risk factors of SVT in AP patients are rarely studied and high-quality prospective studies are needed to further verify and improve the findings of this study.
References
- Yang Y, Wang YZ, Li YJ, Yao W, Wang Z, Yang X, et al. Analysis of risk factors for acute pancreatitis with thrombotic diseases. Chin J Pancreatol. 2021; 21: 258-263.
- Gonzelez HJ, Sahay SJ, Samadi B, Davidson BR, Rahman SH. Splanchnic vein thrombosis in severe acute pancreatitis: a 2-year, single-institution experience. HPB (Oxford). 2011; 13: 860-864.
- Harris S, Nadkarni NA, Naina HV, Vege SS. Splanchnic vein thrombosis in acute pancreatitis: a single-center experience. Pancreas. 2013; 42: 1251-1254.
- Xu W, Qi X, Chen J, Su C, Guo X. Prevalence of Splanchnic Vein Thrombosis in Pancreatitis: A Systematic Review and Meta-Analysis of Observational Studies. Gastroenterology Research and Practice. 2015; 2015: 245460.
- Sissingh NJ, Groen JV, Koole D, Klok FA, Boekestijn B, Bollen TL, et al. Therapeutic anticoagulation for splanchnic vein thrombosis in acute pancreatitis: A systematic review and meta-analysis. Pancreatology. 2022; 22: 235-243.
- Anis FS, Adiamah A, Lobo DN, Sanyal S. Incidence and treatment of splanchnic vein thrombosis in patients with acute pancreatitis: A systematic review and meta-analysis. Journal of Gastroenterology and Hepatology. 2022; 37: 446-454.
- Roch AM, Maatman TK, Carr RA, Colgate CL, Ceppe EP, House MG, et al. Venous thromboembolism in necrotizing pancreatitis: an underappreciated risk. J Gastrointest Surg. 2019; 23: 2430-2438.
- Sissingh NJ, Timmerhuis HC, Groen JV, de Jong MJP, Besselink MG, Boekestijn B, et al. Splanchnic vein thrombosis in necrotizing pancreatitis: a post-hoc analysis of a nationwide prospective cohort. HPB (Oxford). 2024; 26: 548-557.
- Zheng J, Han M, Chen J, Deng MM, Luo G. Predictive value of D-dimer and fibrinogen degradation product for splanchnic vein thrombosis in patients with severe acute pancreatitis: a single-center retrospective study. Scand J Gastroenterol. 2023; 58: 1166-1172.
- Yang WX. Early risk predictors identification and predictive value comparison of acute pancreatitis complicated with splanchnic vein thrombosis. Shijiazhuang: Hebei Medical University. 2023.
- Wei ZG. Study on risk factors of portal vein system thrombosis in early stage of acute pancreatitis. Urumqi: Xinjiang Medical University. 2023.
- Yang X, Chen C, Wang LM, et al. Values of Balthazar CT score and D-dimer in predicting acute pancreatitis complicated by portal venous system thrombosis. China Journal of Emergency Resuscitation and Disaster Medicine. 2022; 17: 1198-1201.
- K T, Chan SJ, Varghese C, Lim WB, Cheemungtoo GM, Akter N, et al. A selective anticoagulation policy for splanchnic vein thrombosis in acute pancreatitis is associated with favorable outcomes: experience from a UK tertiary referral center. HPB (Oxford). 2022; 24: 1937-1943.
- Huang WQ, Zhang P, Tao C, et al. Predictive value of modified CT Severity Index Score for splanchnic venous thrombosis in early-stage acute pancreatitis. Chinese Journal of CT and MRI. 2021; 19: 89-91.
- Bjornsson E, Kalaitzakis E, Baldursdottir MB. Splanchnic vein thrombosis in acute pancreatitis is associated with a favorable prognosis: a population based study. Gastroenterology. 2020; 158: 336-336.
- Ling ZY, Chen MF, Cheng W, et al. Risk factors analysis of portal vein thrombosis in severe acute pancreatitis. Chinese Journal of Digestive Surgery. 2020; 19: 1300-1304.
- Ding L, Deng F, Yu C, et al. Portosplenomesenteric vein thrombosis in patients with early-stage severe acute pancreatitis. World Journal of Gastroenterology, 2018; 24: 4054-4060.
- Marra-López C, Concejo FB, Zuleta H, et al. Analysis and Predictive Factors of Splanchnic Vein Thrombosis in Acute Pancreatitis, in a Large National Prospective Cohort. Gastroenterology. 2017; 152: 285-286.
- Fei Y, Gao K, Hu J, et al. Predicting the incidence of portosplenomesenteric vein thrombosis in patients with acute pancreatitis using classification and regression tree algorithm. Journal of Critical Care, 2017; 39: 124-130.
- Wang YC, Attar BM, Jaiswal P, et al. Thrombosis of Splanchnic Veins During a First Episode of Acute Pancreatitis: Prevalence and Outcomes. Gastroenterology. 2017; 152: 279.
- Toque L, Hamy A, Hamel JF, et al. Predictive factors of splanchnic vein thrombosis in acute pancreatitis: A 6-year single-center experience. Journal of Digestive Diseases, 2015; 16: 734-740.
- Zhou J, Ke L, Tong Z, et al. Risk Factors and Outcome of Splanchnic venous thrombosis in Patients with necrotizing acute pancreatitis. Thrombosis Research. 2015; 135: 68-72.
- Chinese Pancreatic Surgery Association, Chinese Society of Surgery, Chinese Medical Association. Guidelines for diagnosis and treatment of acute pancreatitis in China(2021). Chinese Journal of Practical Surgery. 2021; 20: 730-739.
- Yang AL, Vege SS. Splanchnic Vein Thrombosis in Acute Pancreatitis: A Study in 967 Recent, Direct, Consecutive Admissions at a Tertiary-Care Institution. Gastroenterology. 2015; 148: 684.
- Dorffel T, Wruck T, Ruckert RI, et al. Vascular complications in acute pancreatitis assessed by color duplex ultrasonography. Pancreas. 2000; 21: 126-133.
- Chooklin S, Pidhirnyy B, Osmilovska I, et al. Risk factors of portal vein thrombosis in acute pancreatitis. Pancreatology. 2016; 3: 91-92.
- Anand A, Gunjan D, Agarwal S, et al. Vascular complications of chronic pancreatitis: A tertiary center experience. Pancreatology. 2020; 20: 1085-1091.
- Vujasinovic M, Dugic A, Nouri A, Brismar TB, Baldaque-Silva F, Asplund E, et al. Vascular Complications in Patients with Chronic Pancreatitis. Journal of Clinical Medicine. 2021; 10: 3720.
- Kasuda S, Nishiguchi M, Yoshida S, Ohtsu N, Adachi N, Sakurai Y, et al. Enhancement effect of ethanol on lipopolysaccharide-induced procoagulant status in human umbilical endothelial cells. Soudni lekarstvi. 2009; 54: 44-48.
- Licheri D, Vargiu R, Del MS, Fadda F, Mancinelli R. Chronic ethanol consumption induces hypomotility in the portal vein of Sardinian alcohol-preferring rats. Alcohol & Alcoholism. 1999; 34: 169-174.
- Pandey V, Patil M, Patel R, Chaubal A, Ingle M, Shukla A. Prevalence of splenic vein thrombosis and risk of gastrointestinal bleeding in chronic pancreatitis patients attending a tertiary hospital in western India. Journal of Family Medicine & Primary Care. 2019; 8: 818-822.
- Rebours V, Boudaoud L, Vullierme MP, Vidaud D, Condat B, Hentic O, et al. Extrahepatic portal venous system thrombosis in recurrent acute and chronic alcoholic pancreatitis is caused by local inflammation and not thrombophilia. Am J Gastroenterol. 2012; 107: 1579-1585.
- Severinsen MT, Kristensen SR, Johnsen SP, Dethlefsen C, Tjonneland A, Overvad K. Smoking and venous thromboembolism: a Danish follow-up study. Journal of Thrombosis and Hemostasis. 2009; 7: 1297-1303.
- Robbins AJ, Lusczek E, Bellin MD, Benner A, Alwan FS, Beilman G. Thromboembolic Complications in the First Year After Acute Pancreatitis Diagnosis. Pancreas. 2021; 50: 751-755.
- McGuire S, Maatman T, Allen S, et al. Splanchnic venous thrombosis in necrotizing pancreatitis: common, heterogenous, and deadly. HPB. 2021; 23: 500.
- Roch AM, Maatman TK, Carr RA, Colgate CL, Ceppa EP, House MG, et al. Venous Thromboembolism in Necrotizing Pancreatitis: an Underappreciated Risk. J Gastrointest Surg. 2019; 23: 2430-2438.
- Li RC, Zhang JL, Li R, Zhang Y, Zhu J, Dong W, et al. Clinical features and prediction of 152 patients of acute pancreatitis complicated with portal vein system thrombosis. Chin J Dig. 2021; 41: 29-34.
