
Case Report
Austin Gynecol Case Rep. 2017; 2(2): 1013.
Postradiogenic Atypical Vascular Lesion of the Vulva in a Young Woman after Treatment for Cervical Cancer - Case Report and Review of the Literature
Schubert M¹, Kosse J¹, Braun S², Guthier C³ and Jackisch C¹*
¹Department of Obstetrics and Gynecology, Sana Klinikum Offenbach, Offenbach Germany
²Department of Pathology, Sana Klinikum Offenbach, Offenbach Germany
³Gynecologic Practice, Offenbach, Germany
*Corresponding author: Christian Jackisch, Department of Obstetrics and Gynecology, Sana Klinikum, Offenbach, Starkenburgring 66, D-63069 Offenbach, Germany
Received: January 31, 2017; Accepted: March 13, 2017; Published: March 22, 2017
Abstract
A 43-year-old caucasian women suffers from an extensive and painful vulvar lesions. Prior to this presentation the patient was treated for cervical cancer with surgical treatment and radio chemotherapy seven years ago. A malignant and infectious cause of these lesions was excluded and Atypical Vascular Lesion (AVL) of the vulva was diagnosed by histopathology. AVL is defined as benign vascular proliferation with widened atypical vessels interepidermal and in the deeper dermis. These lesions cause multiple small papules and are mainly located in the radiation field after cancer treatment.
AVL embraces a histological spectrum that ranges from banal appearing lesions closely resembling a lymphangioma circumscriptum to capillary vascular proliferations with nuclear atypia resembling an angiosarcoma. The patient was treated with a radical skinning vulvectomy for a complete resection of the altered tissue. The patient did not relapse under a close follow up and her quality of life improved, significantly.
Keywords: Atypical vascular lesion of the vulva; Lymphangioma circumscriptum; Angiosarcoma; Vulvar pain; Lymphatics; Cervical cancer; Radiation; Vulvectomy
Abbreviations
AVL: Atypical Vascular Lesion; CD: Cluster of Differentiation; FISH: Fluorescence In Situ Hybridization; Gy: Gray
Introduction
AVL is a rare disease that can be acquired especially after radiation for cervical cancer therapy induced lymphedema. It presents with multiple cutaneous papules that may cause pain along with other discomforting symptoms. The differential diagnoses are infectious diseases such as genital herpes but also malignant tumours such as angiosarcoma or cutaneous metastasis of a prior cancer.
Case Presentation
Clinical presentation
A 43-year-old caucasian patient presented with a lesion of the vulva, declining over the last four years. She suffered from itching, burning and swelling of the vulva. The clinical examination revealed multiple small, wetted papules covering the complete vulva. The lesions were most prominent confined to the labium majus but had also emerged on the labia minora (Figure 1).
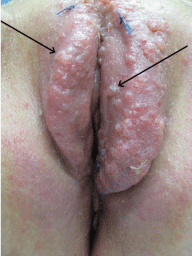
Figure 1: Clinical appearance at first presentation. Multiple small papules
completely covering the labia majora and partly the labia minora. Vulva
appears swollen and reddened. Blue threads where biopsies have been
taken.
The patient is nulliparous and was diagnosed with cervical cancer FIGO IIB seven years prior to her first presentation at our institution with these lesions. The cervical cancer was treated with radical hysterectomy PIVER III including pelvic and para aortic lymph node dissection. Surgery was complicated by a post-surgical ileus with consecutive re-laparotomy resulting in a partial resection of the small bowel. A combined radiochemotherapy with cisplatin and percutan radiation was added due to parametric infiltration followed by vaginal after loading. The total radiation dose was 60, 4 Gy.
Three years after completion of the radiochemotherapy, she noticed first symptoms, which can be ascribed to these lesions. She developed extensive lymphedema of the legs since the treatment of the cervical cancer. Before presentation at our hospital, the lesions had been treated with topical application of aciclovir over a longer period due to the suspected diagnosis of a genital herpes. Adding to this topical treatment excision of bigger lesions and a laser destruction of the other lesions was performed. The pathology revealed a chronic inflammation and no signs of malignancy or recurrence of the cervical cancer. The clinical course did not improve by this therapy.
Histopathology
Several punch biopsies were taken from the vulvar lesions for diagnostic work up. The samples show widened atypical vessels interepidermal and in the deeper dermis. The vessels are lined with a single row of endothel (endothelium) with prominent nuclei (Figure 2c). These endothelial cells showed a strong expression of CD31 and D2-40, partially positive for factor VIII and negative for CD34. CD31 is a widely used vascular endothelial marker that is expressed in all benign and malignant vascular lesions, whereas D2-40 is a monoclonal antibody that reacts with a fixation-resistant epitope on lymphatic endothelium [1].
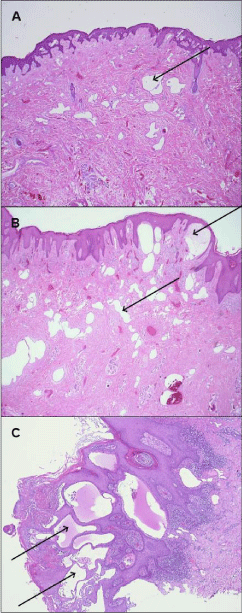
Figure 2: Spectrum of AVL that ranges from the minimal type (A) Variation
with peridermal infiltration (B) Full picture with subepthelial dilated lymphatic
vessels (C) H&E staining.
There were no signs for angiosarcoma or cutaneaous metastasis of the previous cervical cancer. The woman was diagnosed with a secondary atypical vascular lesion of the vulva of the lymphatic type, which is most likely a post radiogenic alteration.
Surgical treatment and follow up
The rationale for our treatment was the complete resection of the altered tissue. Therefore, a radical skinning-vulvectomy with partial removal of the subcutaneous fat tissue was performed. The clitoris could be preserved due to macroscopic normal skin, whereas the labia minora had to be removed (Figure 3). In addition several skin-biopsies were obtained from the mons pubis and lower abdomen as there were lesions that looked like AVL. The biopsies were unremarkable for AVL in these areas. The defect was covered with EpiGard™ (biovision), a synthetic skin substitute, for a week until a skin graft could be placed after we received an unremarkable complete histopathologic work up of the tissue removed.
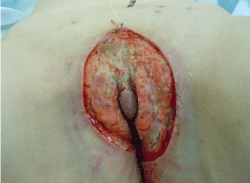
Figure 3: Intraoperative situs after clitoris preserving skinning vulvectomy.
The defect was covered with a synthetic skin substitute until a skin graft was
placed one week later.
The postsurgical period was unremarkable. The margins were epithelialized quickly. The leg edema improved with some intense lymphatic drainage in the postsurgical period. Three months after the surgery, a small papule was first noted in the area around the clitoris. The patient had no signs and symptoms of the AVL compared to the pre-surgical period. The lesion was first monitored, then biopsied without any signs for recurrence.
After a follow period of almost one year her quality of life improved substantial, only some itching remained as a relevant symptom. No pain or any other discomforting symptoms were reported. Micturition and bowel movements were unremarkable. The clinical examination after one year revealed an extensive amount of scar tissue with a stenosis of the vaginal introitus. The small papules were clinically asymptomatic and stable in size (Figure 4). Up to now, she is unremarkable on a three month follow up programme at her gynecologist.
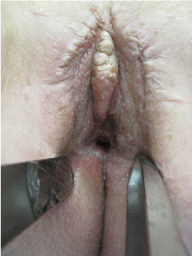
Figure 4: Presentation 12 month after surgery: no sign of AVL with a stenosis
of the vaginal introitus.
Discussion
The AVL of the vulva can be divided into a primary and a secondary form. To our knowledge the etiology of the primary lesion is still unknown, so far. The secondary form has been described as the implication of several different etiologies. It is mainly described following cervical cancer with surgery and/or radiation, can also be caused by infections (sexually transmitted diseases, genital tuberculosis) and others [2]. This disease can be divided into two types: a more common Lymphatic Type (LT) and a Vascular Type (VT) [3].
The clinical presentation of AVL shows multiple cutaneous pinkbrown papules smaller than 1 cm. They are circumscribed intradermal vascular lesions and are mainly multifocal [4]. AVL is sharply defined vascular lesions with culmination subepithelial and in the dermis. They are benign and consist of partly dilated, partly confluenting lymphatic vessels and endothelial cells that can focally be atypical.
The AVL most prominently involves the superficial dermis but can extend into the deep dermis and the subcutaneous layer [5] (Figure 2a,2b). The histological spectrum of AVL is arranged in between the lymphangioma circumscriptum and the angiosarcoma. Therefore the resembling and confusion of atypical vascular lesions with both lesions can be explained by the development of the atypical vascular lesions - it is a continuum that results from a disruption of a previously normal lymphatic system [6].
A fibrosis with lymphangiectasia may alter to a lymphangioma circumscriptum. The lymphangioma circumscriptum is typically asymptomatic unless it is bothering the patient. It is composed of dilated lymphatic channels with thick walls that extend into the epidermis and dermis and there can be a stromal infiltration of lymphocytes. This lesion can progress to an atypical vascular lesion with the features described above (Figure 2a,2b,2c). Finally, atypical vascular lesion may progress to an angiosarcoma, which might explain the overlap in their histological features [4].
AVL therefore has to be differentiated carefully: The separated vessels are lined by flattened or slightly protuberant lymphatic endothelium, express CD31, D2-40 (podoplanin), and variably CD34. MYC amplification by FISH and nuclear expression of MYC can be used to discriminate a post-irradiation angiosarcoma, as it does not amplify in AVL. Nuclei may appear hyperchromatic, but not enlarged or with irregular shape. Progressive lymphangioma shows infiltrating vessels more extensively within the dermis [3,5,6].
To the best of our knowledge, there is only one case report reporting an AVL after radiation for breast cancer with a final progression into an angiosarcoma. This report supports the assumption of AVL being a precursor for angiosarcoma, which is still unclear at present [7]. Due to the patient’s history of cervical cancer, cutaneous metastasis had to be ruled out by histopathology. There have been reported cases of the very unusual clinical presentation of cutaneous metastasis that resembled that of lymphangioma circumscriptum. Genital herpes and other infectious diseases have to be ruled out by clinical work up [8].
AVL arise mainly in the radiation field. There are cases of atypical vascular lesions in the larynx after radiation. Research in those fields revealed the typical interval of almost three years after radiation and an association with the irradiation dose of 40-60 Gy. There is no known correlation for the occurrence of AVL of the vulva [9].
Surgery is the treatment of choice for AVL. Nonsurgical interventions, such as laser vaporisation, cryotherapy, or topical administration of bleomycin, have been used in order to avoid surgery-related complications, but the results are not satisfactory [10]. There is some evidence that a surgery can be also complicated by a high incidence of recurrence, local nerve damage and extensive scar tissue but as the patients suffer from very distressing symptoms, it seems worth to consider surgery for these women if a complete removal is feasible and they are motivated for a close clinical followup over a longer period of time.
The clinical challenge is the diagnosis and the differentiation between AVL and angiosarcoma. The awareness for this rare and benign, but very distressing disease should be raised and the patients at risk should be identified early on to avoid and prevent a long period suffering from signs and symptoms with negative impact of their quality of life.
References
- Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomas. Modern Patholology. 2002; 15: 434-440.
- Kokcu A, Sari S, Kefeli M. Primary vulvar lymphangioma circumscriptum: A Case Report and Review of literature. Journal of Lower Genital Tract Disease. 2015; 19: e1-e5.
- Fernandez AP, Sun Y, Tubbs RR, Goldblum JR, Billings SD. FISH for MYC amplification and anti-MYC immunohistochemistry: useful diagnostic tools in the assessment of secondary angiosarcoma and atypical vascular proliferations. Journal of Cutaneous Pathology. 2012; 39: 234-242.
- Goldblum JR, Weiss SW, Folpe AL. Soft tissue tumors. 6th edn. Elsevier. 2014.
- Calonje J, Brenn T, Lazar A, McKee P. McKee’s Pathology of the Skin. 4th edn. Elsevier. 2011.
- Stewart C, Chan T, Platten M. Acquired lymphangiectasia (‘lymphangioma circumscriptum’) of the vulva: a report of eight cases. Pathology. 2009; 41: 448-453.
- Patton K, Deyrup A, Weiss S. Atypical Vascular Lesions after Surgery and Radiation of the Breast: A Clinicopathologic Study of 32 Cases Analyzing Histologic Heterogeneity and Association with Angiosarcoma. American Journal of Surgical Pathology. 2008; 32: 943-950.
- Kim W, Park H, Kim H, Kim S, Ko H, Kim B, et al. Vulval Metastasis from Squamous Cell Carcinoma of the Cervix Clinically Presenting as Lymphangioma Circumscriptum. Annals of Dermatology. 2011; 23: 64-67.
- Mudaliar K, Borrowdale R, Mehrotra S. Post-radiation Atypical Vascular Lesion/Angiosarcoma Arising in the Larynx. Head and Neck Pathology. 2014; 8: 359-363.
- Ahn S, Chang S, Choi J, Moon K, Koh J, Kim S. A case of unresectable lymphangioma circumscriptum of the vulva successfully treated with OK-432 in childhood, Journal of the American Acadamy of Dermatology. 2006; 55: S106-S107.