
Case Report
Austin Gynecol Case Rep. 2022; 7(2): 1033.
Case Report of Insular Carcinoid Arising From Mature Cystic Teratoma
Vesna A¹, Siandra S¹, Eva SB¹*, Neli B², Nezhla S¹ and Drage D¹
¹University Clinic of Obstetrics and Gynecology, Skopje, R Macedonia
²University Clinic of Oncology and Radiology, Skopje, R Macedonia
*Corresponding author: Sozovska Belchovska Eva University Clinic of Obstetrics and Gynecology, Skopje, R Macedonia
Received: November 07, 2022; Accepted: December 16, 2022; Published: December 22, 2022
Introduction
Primary ovarian Neuroendocrine Tumors (NETs) develop in pure form or in association with other tumors, mainly teratomas. Teratomas are the most common type of ovarian germ cell tumor. They are divided into three categories: mature (cystic or solid, benign), immature (malignant), and monodermal or highly specialized. Most teratomas are cystic and composed of mature adult-type tissues; they are better known as dermoid cysts. The Mature Cystic Teratoma (MCT) accounts for more than 95 percent of all ovarian teratomas and is almost invariably benign [1]. Mature cystic teratomas contain mature tissue of ectodermal (e.g, skin, hair follicles, sebaceous glands), mesodermal, and endodermal origin [2]. They are bilateral in 10 to 17 percent of cases [4]. Malignant transformation occurs in 0.2 to 2 percent of mature cystic teratomas [5-7]. Mature teratomas with malignant transformation comprise 2.9 percent of all malignant OGCTs [8]. The most common malignant change in a dermoid cyst is squamous cell carcinoma, followed by adenocarcinoma and carcinoid tumor [9-11]. The prevalence of Primary Ovarian Carcinoids (POC) is merely 0.1% in ovarian neoplasms and 1% in carcinoid tumors. POC was classified into trabecular, strumal, mucinous, and insular types, among which the latter is the most prevalent type and the only 1 associated with Carcinoid Syndrome (CS.)
We report an extremely rare case of insular carcinoid tumor arising from a mature cystic teratoma with typical clinical manifestation.
Case Presentation
A.Z. is 63 years old patient, admitted at the University Clinic of Obstetrics and Gynecology in Skopje, Republic of Macedonia. The patient firstly was referred to the Clinic two months before admission when the problem was initially detected. Transvaginal ultrasound findings done at the first visit showed tumor on the right ovary (10 cm gross), mainly cystic (2/3) with solid parts (1/3), papillary vegetations (max 2, 4cm) and presence of ascites in the abdominal and pelvic cavity. On the (Figure 1) we represent the ultrasound findings.
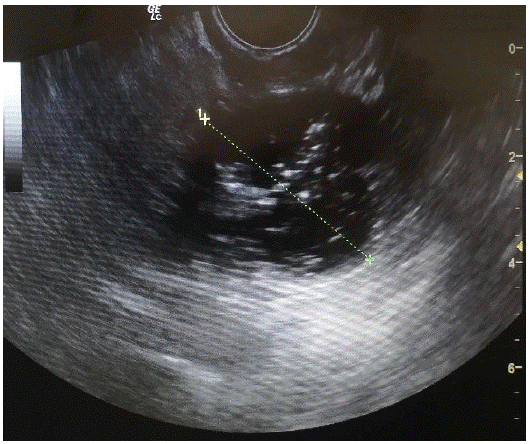
Figure 1: Ultrasound finding of the right ovarian tumor.
The value of tumor marker CA125 was 228 U/ml. The patient had uncontrolled Diabetes mellitus type 2 with a peripheral vascular disease as major complication expressed with bilateral crural ulcerations. The patient also underwent echocardiography and the findings concluded right ventricular volume overload with atrium septum defect, as well as significant tricuspid regurgitation and pulmonary arterial hypertension. Abdominal ultrasound performed by the gastroenterologist showed diffuse enlargement of the liver and moderate congestion in the hepatic veins. Our hospital’s consilium consisting of a gynecologist, an anesthesiologist and an oncologist decided that the patient should undergo thorough pre-operative preparing in means of regulating the diabetes mellitus, vascular disease and heart problems.
On her second admission to our Clinic, during preoperative preparations, it was noticed that the patient had flushing, pre-sented as various skin discolorations, from intense blushing, to deep red or purple hue, on the face, neck, hands and feet, as a sudden event. These episodes continued after surgery, with different time durations (approximately 5-7 postoperative days). Preoperatively, several times the flushing turned into cyanosis, as a characteristic bluish skin spots on the described areas. She had her peripheral vascular disease expressed more than two months ago. The level of tumor marker CA125 was raised to 451 U/ml.
After complete preoperative preparations, the patient underwent surgery. Left adnexectomy and right oophorectomy was performed, as well as omental and peritoneal biopsy, and sample of peritoneal washing was taken for complete surgical staging. Hysterectomy wasn’t performed because of the unstable hemodynamic and heart condition of the patient during surgical treatment, as well as the local surgical difficulties, i.e. extreme fixation of the uterus downwards into the pelvic floor and extreme dilatation of the vein plexuses into the left parametra, which were predictive for possible intra-operative hemorrhage. Second postoperative day the patient became very agitated, confused and with noticeable flushing episodes. Vital parameters didn’t show any signs of hemorrhage, which could lead to the mentioned symptoms. A psychiatrist was called, an acute psychosomatic disorder was diagnosed and risperidone, diazepam and promazine were prescribed to the patient. Also there was gangrene of the left foot, which was treated by a plastic surgeon during the hospitalization. The patient went back home six days after surgical treatment in a stable condition.
Pathological findings: Macroscopically the tumor measured 11x10x7cm and weighted 425g, partly cystic, partly solid. At the cut surface, the cystic part semisepted, unilocular with maximal diameter of 6cm, fulfilled with dense, greasy and yellowish colored liquid with hair, tooth and sebum. The solid part was with maximal diameter 7cm. microscopically the samples had an appearance similar to that of carcinoid tumors of insular type; solid nests of small, round cells. Immunohistochemically, NSE, Chromogranin, Synaptophisin, Leu 7, CKLMW, CK19 were positive for the tumor population.
That leaded to a conclusion that it is Carcinoid tumor – Insular type arising in mature cystic teratoma on the right ovary. Staging: pT1c.
The patient was referred to the University Clinic of Oncology and Radiology, where she received additional chaemotherapy
Six months after the surgical treatment, there were no skin discolorations, flushing or cyanosis. The healing process of the trophic crural ulcerations and gangrenous left foot was complete (“restitutio ad integrum”). The level of tumor marker CA125 was 42.5 U/ml.
On the (Figure 2) we represent the ultrasound finding on the 6-month follow-up control. As we can see, there was no relapse of the tumor, and the endometrium had postmenopausal ultrasound feature.
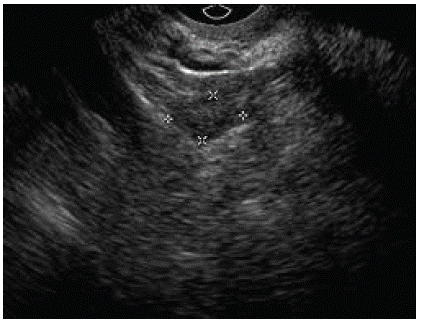
Figure 2A: Left ovary.
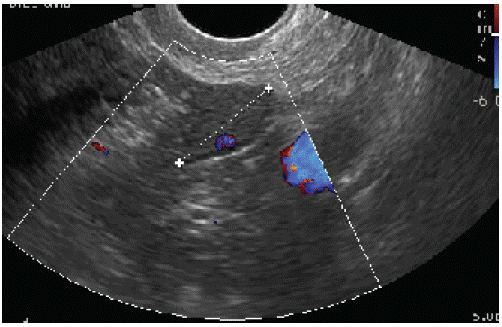
Figure 2B: Right ovary.
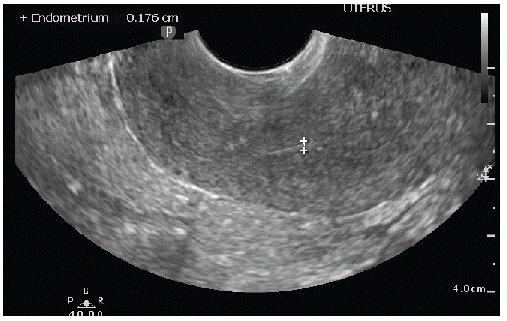
Figure 2C: Uterus with linear endometrium.
Discussion
Carcinoid tumors of the ovary are uncommon, but 150 cases of primary ovarian carcinoids and 40 cases of carcinoid tumors metastatic to the ovary have been reported [12]. Several authors have reported that cystic teratomas constitute 15 to 20% of all ovarian tumors [13]. Although dermoid cysts of the ovary are almost always benign tumors, primary malignancy arising in these cysts rarely encountered, about 1,6% [13,14]. Primary ovarian carcinoid tumors are uncommon and the majority of them are associated with mature cystic teratomas [15]. Robboy et al. divided these tumors into three types: the insular type, trabecular type, and strumal carcinoid type [16]. The insular type is most common, followed by the strumal type. Only the insular type is associated with the carcinoid syndrome [12]. It develops in about one-third of cases, and it can develop without hepatic metastases due to direct venous drainage from the ovary into the systemic circulation. The age incidence of patients with ovarian carcinoids shows a wide range but most patients are postmenopausal [12]. Clinical symptoms of POC are usually not specific. It is difficult to make accurate diagnosis of OC preoperatively. Because of the predominant age group, which is connected with chronic diseases that affects the heart, cardiovascular system, as well as Diabetes mellitus and metabolic disturbances, this tumor may take more time to be detected. The catecholamine, serotonin, bradykinin, substance P, histamine are all responsible for the symptoms that occurred in our patient. Our case has an importance from the point of view of caution these cases not to be misdiagnosed.
References
- Ayhan A, Bukulmez O, Genc C, Karamusrel BS, Ayhan A. Mature cystic teratomas of the ovary: case series from one institution over 34 years. Eur J Obstet Gynecol Reprod Biol. 2000; 88: 153.
- Di Saia PJ, Creasman WT. Germ cell, stromal and other ovarian tumors. In: Clinical Gynecologic Oncology. 7th, Mosby-Elsevier. 2007: 381.
- Caspi B, Lerner-Geva L, Dahan M, Chetrit A, Modan B, et al. A possible genetic factor in the pathogenesis of ovarian dermoid cysts. Gynecol Obstet Invest. 2003; 56: 203.
- Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg HR, et al. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. Lancet Oncol. 2008; 9: 1173.
- Westhoff C, Pike M, Vessey M. Benign ovarian teratomas: a population- based case-control study. Br J Cancer. 1988; 58: 93.
- Comerci JT Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994; 84: 22.
- Singh P, Yordan EL, Wilbanks GD, Miller AW, Wee A. Malignancy associated with benign cystic teratomas (dermoid cysts) of the ovary. Singapore Med J. 1988; 29: 30-4.
- Smith HO, Berwick M, Verschraegen CF, Wiggins C, Lansing L, et al. Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol. 2006; 107: 1075-85.
- Climie ARW, Heath LP. Malignant degeneration of benign cystic teratomas of the ovary. Review of the literature and report of a chondrosarcoma and carcinoid tumor. Cancer 1968; 22: 824-32.
- Hirakawa T, Tsuneyoshi M, Enjoji M. Squamous cell carcinoma arising in a mature cystic teratoma of the ovary. Clinicopathological and topographic analysis. Am J Surg Pathol. 1989; 13: 397-405.
- Palmer PE, Bogojavlensky S, Bhan AK, Scully RE. Prolactinoma in wall of ovarian dermoid cyst with hyperpolactinemia. Obstet Gynecol. 1990; 75: 540-43.
- Talerman A. Carcinoid tumors of the ovary. J Cancer Res Clin Oncol. 1984; 107: 125-35.
- Fukuda T, Ohnishi Y, Terashima T, Iwafuchi M, Itoh S. Peptide Tyrosine- positive ovarian carcinoid tumor arising from a dermoid cyst. Acta Pathologica Japonica. 1991; 41: 394-400.
- Davis KP, Hartmann LK, Keeney GL, Shapiro H. Primary ovarian carcinoid tumors. Gynecol Oncol. 1996; 61: 259-65.
- Scully RE. Carcinoid. In Tumors of the Ovary and Maldeveloped Gonads (Atlas of Tumor Pathology, 2nd series, Fascicle 16). Aimed Forces Institute of Pathology, Washington, D.C. 1979: 274-283.
- Robboy SJ, Scully RE, Norris HJ. Primary trabecular carcinoid of the ovary. Obstet Gynecol. 1977; 49: 202-207.