
Case Report
Austin Gynecol Case Rep. 2023; 8(1): 1037.
Anastrozole is not Always Successful in Preventing Endometrial Hyperplasia in Patients with Estrogen (+) Breast Cancer – Case Report
Antovska V, Dabeski D, Islami NS, Sehfulahi S, Shabani A, Sozovska E* and Malahova I
University Clinic for Gynecology and Obstetrics, Skopje, Republic of North Macedonia
*Corresponding author: Sozovska EUniversity Clinic for Gynecology and Obstetrics, Skopje, Republic of North Macedonia.
Received: January 13, 2023; Accepted: February 13, 2023; Published: February 20, 2023
Abstract
Breast cancer is the most common malignancy among women. Anastrozole is the recommended therapeutic for long-term postoperative therapy in postmenopausal women with estrogen (+) breast cancer. Its advantage over Tamoxifen lies in the fact that it does not have a stimulating effect on the proliferation of the endometrium, and therefore does not represent a risk for the development of endometrial atypia and endometrial cancer. However, it is not always 100% effective in suppressing endometrial proliferation and anaplasia. Our case shows exactly the same situation. In our case, endometrial atypia was diagnosed despite Anastrazole therapy for estrogen (+) breast cancer. The lesson that can be drawn from our case is that cases that are on long-term hormonal therapy with Anastrazole due to previous estrogen (+) breast cancer should be vigilantly monitored for the possible development of premalignant and malignant endometrial changes, including ultrasound gynecological examination at least once/6 months and, if necessary, performance of fractional exploratory curettage.
Keywords: Anastrazol; Estrogen (+) breast cancer; Endometrial hyperplasia
Introduction
There are three biologic subgroups of Breast Cancer (BC): 1) those that express the Estrogen Receptors (ERs), 2) those that express the Human Epidermal growth factor Receptor 2 (HER2), 3) triple-negative BC for: ERs, Progesterone Receptors (PRs); HER2.
In postmenopause, the estrogen production in ovarian granulosa cells stops due to exhaustion of the primordial follicles. In this period, the ovarian stroma, including theca cells, continues the androgens production. Additionally, the adrenal glands produce androgens for the rest of life. All these androgens undergo conversion to estrogens in adipose tissue and negatively affect the prognosis of BC, including disease recurrence. Most authors recommend hysterectomy and surgical, rather than radiological or hormonal castration, because it completely eliminates the ovarian stroma as an estrogen production site. At the same time, the risk of developing Endometrial Cancer (EC) and Ovarian Cancer (OC), which have an increased incidence in cases of breast cancer with gene family mutations (BRCA1 and BRCA2 syndrome), would be eliminated. For patients for whom surgical castration is unacceptable, long-term hormonal therapy is recommended. The drug of choice is an inhibitor of the aromatase enzyme, which is responsible for the conversion of androgens into estrogens in adipose tissue. The selective estrogen receptor modulator, such as Tamoxifen is contraindicated in postmenopausal women, because it has a proliferative effect on the endometrium, including the anaplasia and EC.
Anastrozole is an effective and well tolerated hormone therapy for postmenopausal patients with ERs(+) early BC. Longer follow-up is required for a final benefit risk assessment. We should always be cautious about its 100% effectiveness in suppressing the endometrium, especially since more than 10% of ERs(+) BC enter the group of familial cancers, with familial gene mutation (BRCA1, BRCA 2) and increased risk of EC.
Our case shows this situation. We present a postmenopausal patient with ERs(+)BC, who was treated two months with Tamoxifen, and then therapy has been changed to Anastrazole. However, despite the 1-year Anastrazole therapy, the patient was diagnosed with endometrial atypia, for which a hysterec tomy and bilateral adnexectomy was performed. The question arises whether this atypia existed before the start of Anastrazole therapy or whether it occurred during this therapy. The lesson that can be drawn from our case is that cases that are on long-term hormonal therapy should be vigilantly monitored for the possible development of premalignant and malignant endometrial changes, including ultrasound gynecological examination at least once/6 months and, if necessary, performance of fractional exploratory curettage.
Case Report
A 76-year-old patient who had a radical mastectomy due to BC 14 months ago. The tumor was highly positive for ER(+++), positive for PR (+++) and negative for HER2. The patient had a positive family history of BC from the first relative-mother. Among the other diseases, tere were present: gluten allergy, hypertension, lung emphysema. Menarche occurred at the age of 14, menopause at the age of 50 (she was already 26 years old in postmenopause), BMI was 29. She had 3 previous deliveries and 3 breastfeedings. Immediately after mastectomy, she received a selective estrogen receptor modulator (Tamoxifen) for 2 months, and then the therapy was changed to aromatase inhibitor (AI)-Anastarazol, which she received for 1 year. A control ultrasound examination revealed a very thickened endometrium-14 mm, suspicious for an endometrial polyp.
Hysteroscopy with polypectomy and fractional exploratory curettage was performed. Histopathology analysis of the operative specimen was: endometrial polyp covered by endometrium with mild to moderate atypia, and surrounding endometrium with simplex and complex hyperplasia.
Because of abovementioned histopathology, a hysterectomy with bilateral adnexectomy was immediately performed. Histopathology analysis of the uterus revealed presence of endometrium consisting of multiplied, variable in shape and size, densely packed endometrial glands with glandular morphology of simplex and complex hyperplasia and foci of atypia of mild and moderate degree, as well as presence of two fibroids.
Discussion
Agorastos [1], investigating the efficacy of long-term treatment (12 months) with Anastrazol for endometrial hyperplasia ((4 simple, 5 complex and 2 atypical) in 11 obese postmenopausal women with high operative risk, revealed atrophical endometrium in all 11 patients on the follow-up curettage. AlZaabi [2] examined 204 patients diagnosed with ERs(+)BC regarding the endometrial assessment during tamoxifen or letrozole therapy. Increased endometrial thickness was reported in 8% of the premenopausal and 14% of the postmenopausal group. Other detected endometrial pathologies were: inactive endometrium (1.47%), atrophic endometrium (1.47%), serous carcinoma (0.50%), EC (0.98%), chronic endometritis (0.50%), and they were not significantly associated with tamoxifen or letrozole therapy duration. Two patients developed EC and both are postmenopausal, older than 60. Woolas [3] reported for patient who developed an ERs(+) BC two years after hysterectomy for EC. She received adjuvant Tamoxifen for 2 years and developed a vaginal recurrence of EC.
Aromatase, the enzyme that converts androgens to estrogens, is present in the ovaries (granulose cells), placenta (syncytiotrophoblast), adipose tissue. Studies shown that EC express higher levels of aromatase compared to normal endometrium, hypothesized to function through a paracrine mechanism. Aromatase inhibitors act to block the action of the enzyme, and have been show to suppress up to 95-98% of circulating estrogens [4]. The results of Chlebowski` study [5] suggested that AIs, compared to Tamoxifen, lower circulating estrogens in BC patients and with that might reduce the risk of EC. A recent meta-analysis by the Early Breast Cancer Trialists Collaborative Group(EBCTCG) [6] on AIs vs. Tamoxifen for hormone therapy for BC, showed a reduced EC incidence in the AIs-group (10-year incidence 0.4% vs 1.2%; RR=0.33, 95% CI; 0.21-0.51).
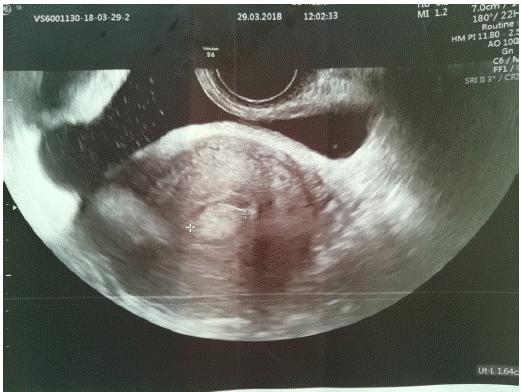
Figure 1: Preoperative ultrasound present clearly visible endometrial polyp.
The prevention study IBIS II [7], showed that the use of Anastrozole in postmenopausal women with family history or previous mastectomy for in situ ductal ERs(+)BC DCIS, reduced significantly the risk of BC and EC (for 5-year median follow-up of 5 years, 3 cases of EC in Anastrozole-group vs. 5 with placebo [HR= 0.61 (95% CI; 0.15-2.54).
Bogliolo [8] performed a systematic review to assess the effectiveness of AIs in the treatment of advanced EC. The potential clinical benefit of AIs in advanced and recurrent EC was underlined, highlighting the need to investigate the biological characteristics of tumor, such as hormone receptors. According to Laura Paleari and Andrea DeCensi, [9] evaluation of ERs/PRs expression should be routinely performed in endometrial cancer to guide treatment decisions.
There are several possible scenarios that can explain the cause of endometrial atypia in our patient despite 1-year Anastazol therapy: 1). Endometrial changes may have existed before the start of treatment with Anastrazole, that is, the Endometrial Atypia (EA) occurred during the 2-month treatment with Tamoxifen. 2). endometrial atypia may have existed at the time of occurring BC. 3). the patient maybe was a carrier of a gene family mutation (BRCA 1 or 2), which led to an increased risk of developing two estrogen-dependent cancers at the same time, BC and EC. 4). the degree of conversion of androgens into estrogens maybe was significantly high, so the dose of Anastrazol was insufficient to perform a complete inhibition of the aromatase enzyme in adipose tissue, which resulted in the appearance of EA. 5). last possibility is that Anastrozole is not such an effective in preventing of EC, and thus it seems also in preventing the relapse of BC.
Conclusion
We present a case of a postmenopausal patient with ERs(+) BC, who was treated several months with Tamoxifen, and then 1 year with Anastrazole. However, despite the 1 year Anastrasole therapy, she was diagnosed with endometrial atypia, for which a hysterectomy and bilateral adnexectomy was performed. The question arises whether this atypia existed before the start of Anastrazole therapy or whether it occurred during this therapy. The lesson that can be drawn from our case is that cases that are on long-term therapy with Anastrazole due to previous ERs(+) BC should be vigilantly monitored for the possible development of premalignant/malignant endometrial changes, including gynecological ultrasound at least once/6 months and, if necessary, performance of fractional exploratory curettage.
References
- Agorastos T, Vaitsi V, Pantazis K, Efstathiadis E, Vavilis D, et al. Aromatase inhibitor anastrozole for treating endometrial hyperplasia in obese postmenopausal women. Eu J Obs Gynecol and Rep Biol. 2005; 118: 239-40.
- Al Zaabi A, Al Amri H, AL Ajmi G, Allawati M, Muhanna F, et al. Endometrial Surveillance in Tamoxifen and Letrozole Treated Breast Cancer Patients. Cureus. 2021; 13: e20030.
- James Woolas, Megan Davis, Siavash Rahimi. Recurrence of endometrial cancer in a hysterectomised patient treated with tamoxifen for breast cancer: a case report. Journal of the Royal Society of Medicine. 2020; 113: 454-456.
- Folkerd EJ, Dixon JM, Renshaw L, A’Hern RP, Dowsett M, et al. Suppression of plasma estrogen levels by letrozole and anastrozole is related to body mass index in patients with breast cancer. J Clin Oncol. 2012; 30: 2977-2980.
- Chlebowski RT, Schottinger JE, Shi J, Chung J, Haque R, et al. Aromatase inhibitors, tamoxifen, and endometrial cancer in breast cancer survivors. Cancer. 2015; 121: 2147-2155.
- Early Breast Cancer Trialists. Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomized trials. Lancet. 2015; 386: 1341-1352.
- Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014; 383: 1041-1048.
- Bogliolo, Gardella B, Dominoni M, Musacchi V, Cassani C, et al. Effectiveness of aromatase inhibitors in the treatment in the treatment of advanced endometrial adenocarcinoma. Arch Gynecol Obstet. 2016; 293: 701-708.
- Laura Paleari, Andrea DeCensi. Aromatase Inhibitors and Endometrial Adenocarcinoma: The Role of Hormone Receptor Assessment. J Gynecol Women’s Health. 2018; 9: JGWH.MS.ID.555771.