
Case Report
Austin Gynecol Case Rep. 2023; 8(1): 1038.
A Case of BRAF V600E Mutation and Targeted Therapy in Borderline Ovarian Serous Cystadenoma
Min JX and Yuan HY*
Department of Obstetrics and Gynecology, National Clinical Research Center for Obstetric and Gynecologic Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, China
*Corresponding author: Yuan HYDepartment of Obstetrics and Gynecology, National Clinical Research Center for Obstetric and Gynecologic Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences, Peking Union Medical College, Beijing, China Tel: 138-1158-0852, E-mail: Liuhaiyuan_pumc@126.com
Received: February 15, 2023; Accepted: March 22, 2023; Published: March 29, 2023
Abstract
Background: Borderline ovarian serous cystadenoma with 30% BRAF mutation is common in young women. Although surgery is generally the treatment of choice, surgery or repeated surgery can reduce a woman’s fertility and ovarian reserve. Therefore, it is critical to explore other treatment measures besides surgery.
Case Presentation: We herein describe the clinical outcome and significance of a 41-year-old patient with a BRAF V600E mutation in borderline ovarian serous cystadenoma after treatment with a BRAF inhibitor.
Conclusion: A BRAF inhibitor may be effective in patients with borderline ovarian serous cystadenoma with BRAF V600E mutation.
Keywords: BRAF V600E; Targeted therapy; Borderline ovarian cystadenoma; Mutation; Dabrafenib
Introduction
Borderline epithelial ovarian tumor is also known as low-grade malignant potential tumor or atypical proliferation tumor without obvious infiltration [1], and the primary pathological types are serous and mucinous [2]. Although investigators have in recent years detected the expression of an increasing number of abnormal genes in borderline ovarian serous tumors, there are no reports of targeted therapy for the disease. This report outlines a case of borderline ovarian serous cystadenoma with a BRAF V600E mutation, and the subsequent successfully targeted treatment using a BRAF inhibitor.
Case Presentation
We herein describe a 41-year-old woman who underwent right adnexectomy for “right ovarian mass” under laparoscopy in 2013. The postoperative pathology was borderline serous tumor of the right adnexa, with no specific abnormality of the fallopian tube. In 2016, an abnormal ultrasonogram revealed a left cystic solid ovarian mass 3 cm in diameter; and on 11 January 2018, gynecological ultrasonography showed left adnexal mass, which was the same as the imaging result before primary surgery. This suggested the possibility of borderline ovarian tumor recurrence (Figure 1). We detected the somatic mutation c.1799T>A (p. V600E) in the BRAF gene (NM_004333.6) of the patient using Whole-Exome Sequencing (WES) [3]. Peripheral blood samples were used as germline controls, and the sequencing depth at this locus was 64X, with a mutation frequency of 9.38%. We also noted wild-type KRAS, which was consistent with the conclusion that the two oncogenes were mutually exclusive.
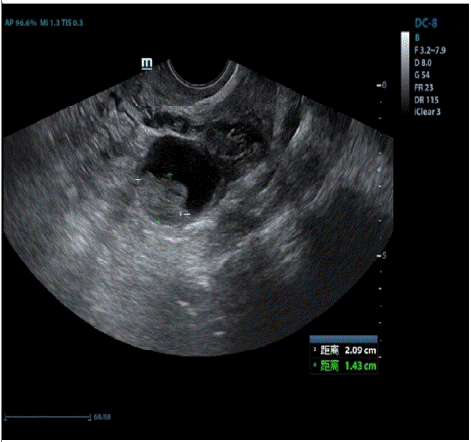
Figure 1:
In May 2018, the patient was administered dabrafenib (a BRAF inhibitor) at a dosage of 50 mg orally twice per day for 3 months, and at follow-up, the tumor size shrank from 4.1cm×2.7cm to 2.9cm×2.7cm×2.6cm. Adverse reactions were grade I hair loss and mild discomfort at the waist, with no other side-effects. The dabrafenib was ultimately halted due to drug cost. After the patient stopped taking it, ultrasonographic examination was rechecked every 3 months. The tumor gradually increased from 3.2cm×2.8cm×2.8cm in Nov 2018 to 4.7cm×3.9cm×3.4cm in Dec 2019. On 9 June 2020, ultrasono graphic examination showed a cystic solid mass of the left adnexa 4.6cm×3.4cm×3.4cm in (Figure 2).
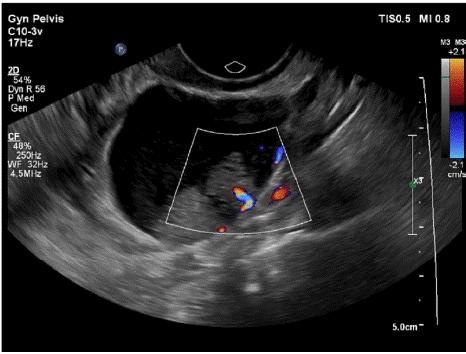
Figure 2:
On 11 June 2020, the woman who had no fertility desire underwent laparoscopic surgery for borderline ovarian tumor-staging, with removal of the entire uterus + left adnexectomy + pelvic lymph-node dissection + omentectomy + appendectomy + adhesiolysis. During the operation, we determined that the left ovarian cyst was enlarged to 5 cm with a clear borderline, but with no tumors on the ovarian surface. The right adnexa were absent, and adhesions were observed in the pelvic cavity. Postoperative pathology showed borderline left ovarian serous cystadenoma, but no tumor cells were found in other tissues such as the omentum or appendix, or in abdominal- or pelvic-lavage fluid.
After surgery, the patient was examined at regular follow-ups by gynecological ultrasonography for 2 years without signs of recurrence.
Methods
Generation of Whole-Exome Sequencing (WES) Data
Genomic DNA From Formalin-Fixed Paraffin-Embedded (FFPE) tumor samples was extracted using a GeneRead DNA FFPE kit (QIAGEN) or Maxwell®FFPE Plus DNA Kit (Promega); and a blood sample from this patient was extracted using a TGuide Blood Genomic DNA Kit (TIANGEN). Then, 0.1–1μg of DNA was sheared into 200–300-bp fragments using a Covaris kit (Covaris, MA, USA), and the resulting DNA fragments were repaired and 3' poly A-tailed. Adapters were ligated to both ends of the fragments, followed by size selection, and the size-selected fragments were amplified by Polymerase Chain Reaction (PCR). We conducted exome capture using the Sure Select Human All Exon V6 platform (Agilent) according to the manufacturer’s protocol, followed by PCR amplification. Libraries were prepared using the KAPA Library Quantification Kit (Kapa Biosystems, MA, USA), and validated DNA libraries were sequenced on an Illumina XTEN or NovaSeq 6000 at a mean sequencing depth for the tumor at 243× and for normal samples at 108×.
Somatic Mutation-Rate Analysis
WES sequencing read pairs were trimmed, and only read pairs with ≤15 N bases and >50% high-quality bases were kept for subsequent analyses. The resulting high-quality reads were aligned to the human reference genome (Homo_-sapiens_assembly19) using the Burrows-Wheeler Aligner (0.7.17) [4]. To improve alignment accuracy, we employed the Genome Analysis Toolkit (version 3.8.1) [5] to process BAM files, which included marking duplicates, and the local realignment around high-confidence insertions and deletions. To call somatic mutations accurately, we used the variant-calling pipeline developed by TCGA MC3 project. Briefly, this pipeline employs five callers to call substitution mutations and three callers to identify small indels, with detailed annotation. We only retained substitution mutations and indels supported by at least two callers for further analyses. All mutations were retained for subsequent analyses if their positions were ≥10× in both normal and tumor samples. To calculate the Tumor-Mutation Burden (TMB) values, we herein used only missense or ORF shift mutations in the overlapping targeted regions with the Sure Select Human All Exon V6 platform and those regions defined in TCGA MC3 project [6]. We exploited MANTIS to call the MSI status for each tumor based on 2539 loci from the mSINGS package [3].
Discussion
Early symptoms of borderline serous cystadenoma of the ovary may be similar to those of benign tumors; ascites can be found in the late stages [7], and over 50% of the pelvic masses in patients are palpable [7]. Many methods are helpful with diagnosis– including gynecological ultrasonography, MRI, and laboratory examination. As the accuracy of intraoperative frozen pathology is 87% [8], surgery has evolved into the most important treatment modality for borderline ovarian tumors. A majority of recurrences entail some types of tumors and can be resected, but adjuvant therapy post-operatively is not effective in controlling disease recurrence.
Types of Genetic Mutations in Borderline Ovarian Tumors
Borderline tumors have not been as widely examined as ovarian cancer with respect to genetic mutations. However, BRAF and KRAS mutations are acknowledged to be the most common in borderline ovarian serous tumors, with rates above 30% and 20% [9], respectively. The two tumor types are mutually exclusive, as wild-type KRAS was observed in all BRAF-mutated tumors, and vice versa [10]. Mutations in other genes such as PIK3CA, BRCA1, EGFR, CTNNB1, PTEN, ERBB2, and AKT2 [11] are relatively infrequent, and reports of them are limited.
BRAF Mutation and its Significance in the Treatment of Other Tumors
BRAF is a protooncogene located on chromosome 7q34 [12], and it encodes a serine- threonine protein kinase that plays an important role in the RAS-RAF-MEK-ERK- signaling pathway. BRAF links receptors and RAS proteins on the cell surface with nuclear transcription factors via MEK and ERK and initiates the participation of a variety of factors in the regulation of numerous biological events in cells–including cellular proliferation, differentiation, and apoptosis. Most mutations in BRAF occur in exon 15, where the thymine at nucleotide 1799 is converted to adenine, resulting in the mutation of valine at codon 600 (V600E) to glutamate [13] and leading to the continuous activation of MEK and ERK. Ultimately, the cells continue to proliferate, and this reflects a critical function in the formation of some tumors such as melanoma, colon adenocarcinoma, and ovarian serous tumors.
The mutation rates of BRAF in primary and metastatic melanoma are approximately 40%–60% and 50% [13], respectively, and the principal BRAF mutation site is BRAF V600E [14]. Current studies have revealed that BRAF mutations in melanoma patients portend lower Overall Survival (OS) and a poorer prognosis than those for patients with wild-type BRAF [14]. In view of the poor prognosis for patients with unresectable or metastatic melanoma and the limited benefits of standard chemotherapy, the emergence of small-molecule BRAF inhibitors has completely transformed the treatment of melanoma. BRAF inhibitors such as vemurafenib and dabrafenib are now used to treat unresectable or metastatic melanoma, with a response rate of approximately 50% [13]. Compared with dacarbazine, BRAF inhibitors such as vemurafenib can increase the OS at 6 months by 20% and reduce the relative risk of death by 63% and the risk of tumor progression by 74% [15]. These same investigators also described a Median Progression-Free Durvival (mPFS) of 5.3 months in the vemurafenib group and 1.6 months in the dacarbazine group[15].
In addition, BRAF mutations have been uncovered in ~10%–15% of colorectal cancers, with the mutat?i on in BRAF V600E being the primary mutation site. This gene is mutated early in colorectal cancer and comprises an independent adverse predictor using multivariate analysis [13] because of its poor response to standard chemotherapeutic drugs and its rapid progression. With further elucidation of the BRAF-signaling pathway, triplet therapy (irinotecan, cetuximab, and vemurafenib) has achieved better rates of PFS (4.4 months vs. 2.0 months), objective remission (16% vs. 4%), and disease-control (67% vs. 22%, respectively) than double therapy (irinotecan and cetuximab) [16].
The aforementioned data showed that BRAF inhibitors achieved favorable therapeutic effect in other tumors, and we therefore also employed dabrafenib as the experimental treatment for this patient; and its therapeutic effect was proven by the clinical reduction in tumor size.
BRAF Gene Mutation in Borderline Ovarian Tumor
The most common BRAF mutation is BRAF V600E, and investigators depicted BRAF mutations as related to specific cell types that showed aging characteristics in borderline ovarian serous tumors [9]; such mutations prevented the disease from developing into malignancy, and thus, the BRAF V600E mutation was positively correlated with borderline ovarian tumor prognosis. However, there was no significant effect of BRAF V600E mutation on five-year survival rate [17]. Only a small number of serous cystadenomas develop into borderline serous tumors, with the cystadenoma epithelium adjacent to the borderline tumor similar to normal ovarian epithelium and lacking cytologic atypia. In addition, BRAF mutation in borderline tumors is also found in adjacent tumor cells. These lines of evidence therefore strongly indicate that BRAF mutation is an early tumor event that precedes borderline tumorigenesis [10]. However, whether the early treatment of BRAF-gene mutation can alter the clinical outcome of patients requires further evaluation, as we showed in our present case report.
A histological diagnosis of borderline ovarian serous cystadenoma was confirmed in the present study by surgery in 2013, and after 3 years of follow-up, a recurrence was detected in the ovary. In 2018, after 3 months of treatment with oral daBRAFenib, the tumor size was reduced significantly from 4.1cm×2.7cm to 2.9cm×2.7cm×2.6cm. However, after the drug was halted due to its cost to the patient, the tumor size gradually increased from 2cm×2.8cm×2.8cm to 4.7cm×3.9cm×3.4cm; and in 2020, we confirmed at surgery that borderline ovarian serous cystadenoma recurred. However, the fact that the tumor size was significantly reduced during the targeted drug therapy indicated that the drug could control borderline ovarian serous cystadenoma.
The population prone to borderline ovarian tumors tends to be younger, with ~1/3 of them being less than 40 years of age [18]. According to a recent study, only 22% of the patients achieved pregnancy after repeated surgery, with only 38% of the late-stage patients undergoing successful pregnancy [19]. Such high infertility rates therefore necessitate fertility preservation, especially for those patients whose recurrence surgery further attenuates ovarian reserve. Repeated surgery might thus be avoided–and ovarian function preserved as much as possible–in patients with BRAF mutations by using BRAF inhibitors to control the disease in such circumstances. We posit that research in this field will be moving in this direction in the near future. However, due to the case reported herein, the effectiveness of this protocol still requires replication in a much larger sample of clinical data.
Conclusion
The use of a BRAF inhibitor may be effective in patients with borderline ovarian serous cystadenoma and who express a BRAF V600E mutation.
References
- Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 1.2020-March 11, 2020. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®).
- Ushijima K, Kawano K, Tsuda N, Nishio S, Terada A, et al. Epithelial borderline ovarian tumor: Diagnosis and treatment strategy. Obstet Gynecol Sci. 2015; 58: 183-187.
- Salipante SJ, Scroggins SM, Hampel HL, Turner EH, Pritchard CC. Microsatellite instability detection by next generation sequencing. Clin Chem. 2014; 60: 1192-1199.
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009; 25: 1754-1760.
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010; 20: 1297-1303.
- Ellrott K, Bailey MH, Saksena G, Covington KR, Kandoth C, et al. Scalable Open Science Approach for Mutation Calling of Tumor Exomes Using Multiple Genomic Pipelines. Cell Syst. 2018; 6: 271-281.e7.
- Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs. 2014.
- Shih KK, Garg K, Soslow RA, Chi DS, Abu-Rustum NR. Accuracy of frozen section diagnosis of ovarian borderline tumor. Gynecol Oncol. 2011; 123: 517-521.
- Zeppernick F, Ardighieri L, Hannibal CG, Vang R, Junge J, et al. BRAF mutation is associated with a specific cell type with features suggestive of senescence in ovarian serous borderline (atypical proliferative) tumors. Am J Surg Pathol. 2014; 38: 1603-1611.
- Ho CL, Kurman RJ, Dehari R, Wang TL, Shih IeM. Mutations of BRAF and KRAS precede the development of ovarian serous borderline tumors. Cancer Res. 2004; 64: 6915-6918.
- Stemke-Hale K, Shipman K, Kitsou-Mylona I, de Castro DG, Hird V, et al. Frequency of mutations and polymorphisms in borderline ovarian tumors of known cancer genes. Mod Pathol. 2013; 26: 544-552.
- Jurca MC, Iuhas OA, Puiu M, Chirita-Emandi A, Andreescu NI, et al. Cardiofaciocutaneous syndrome-a longitudinal study of a case over 33 years: case report and review of the literature. Rom J Morphol Embryol. 2021; 62: 563-568.
- Ritterhouse LL, Barletta JA. BRAF V600E mutation-specific antibody: A review.Semin Diagn Pathol. 2015; 32: 400-408.
- Ny L, Hernberg M, Nyakas M, Koivunen J, Oddershede L, et al. BRAF mutational status as a prognostic marker for survival in malignant melanoma: a systematic review and meta-analysis. Acta Oncol. 2020; 59: 833-844.
- Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, et al. BRIM-3 Study Group. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011; 364: 2507-2516.
- Jin Z, Sinicrope FA. Advances in the therapy of BRAF V600E metastatic colorectal cancer. Expert Rev Anticancer Ther. 2019; 19: 823-829.
- Mayr D, Hirschmann A, Löhrs U, Diebold J. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006; 103: 883-887.
- Palomba S, Zupi E, Russo T, Falbo A, Del Negro S, et al. Comparison of two fertility-sparing approaches for bilateral borderline ovarian tumours: a randomized controlled study. Hum Reprod. 2007; 22: 578-585.
- Jia SZ, Xiang Y, Yang JJ, Shi JH, Jia CW, et al. Oncofertility outcomes after fertility-sparing treatment of bilateral serous borderline ovarian tumors: results of a large retrospective study. Hum Reprod. 2020; 35: 328-339.