
Research Article
Ann Hematol Oncol. 2014;1(1): 1002.
Transformed Follicular Lymphoma: Is Early Autologous Stem Cell Transplantation Indicated in the Modern Era?
Amie E Jackson1, Jingxia Liu2, Nancy L Bartlett3 and Amanda F Cashen3*
1Division of Hematology and Medical Oncology, Mayo Clinic, USA
2Division of Biostatistics, Washington University School of Medicine, USA
3Division of Oncology, Washington University School of Medicine, USA
*Corresponding author: Amanda F Cashen, Division of Oncology, Washington University School of Medicine, 660 South Euclid Ave, Box 8007, St. Louis, MO63110, USA
Received: August 04, 2014; Accepted: August 27, 2014; Published: August 29, 2014
Abstract
Transformation of follicular lymphoma (FL) is associated with an aggressive course and rapid decline in survival. Inclusion of auto logous stem cell transplant (auto-SCT) in the initial treatment of transformed FL (tFL) has been investigated; but most studies pre-date the widespread use of rituximab. The role of early auto- SCT in the current immunotherapy era is not established. This study reports the outcomes of 105 patients with tFL treated at a single institution between 1994 and 2009. 24 patients underwent auto-SCT in first remission. Rituximab was part of the initial therapy for tFL in 86%, and 56% were chemotherapy naïve at transformation. Median follow-up was 44 months. The 2-year and 5-year overall survival (OS) rates for all patients were 78% and 65%, respectively. Survival did not differ among those patients who underwent auto-SCT (PFS 42% and OS 74% at 5 years) compared to those who did not (PFS 30% and OS 63% at 5 years). Age over 60 was associated with worse OS. In conclusion, we did not observe a benefit in PFS or OS when auto-SCT was included in the initial therapy of tFL.
Keywords: Autologous stem cell transplantation; Diffuse large B-cell lymphoma; Follicular lymphoma; Transformed lymphoma; Rituximab
Introduction
Follicular lymphoma (FL) is an indolent malignancy with a historic median overall survival (OS) of 8-12 years, and even better outcomes with modern therapies [1-4]. Transformation to an aggressive lymphoma, most commonly diffuse large B-cell lymphoma (DLBCL), can occur at any point in the disease course. A recent review estimates the risk of clinically significant transformation as 20%at 5 years and 30%at 10 years [5].
Prognosis following transformation is poor, with a historical median survival of less than 1 year [6-8]. Recent studies report modestly longer median OS rates, likely reflecting new treatment options, especially rituximab, as well as better supportive care [2,9- 15]. The subset of patients with transformed FL (tFL) who achieve complete remission (CR) following therapy have a prolonged median OS of40 to 81 months [2,7,10-15]. There is no reproducible prognostic index for tFL. Variably predictive factors include no prior chemotherapy [15], limited stage disease [9], low international prognostic index (IPI), and age less than 60 years [12]. The relatively small population of tFL patients has precluded prospective trials, and patients are frequently excluded both from FL and DLBCL studies. Consequently, treatment is usually based on results of de novo DLBCL trials. As with FL, the addition of rituximab improves outcomes in patients with both de novo and transformed DLBCL, and R-CHOP (rituximab, Cyclophosphamide, adriamycin, vincristine, prednisone) is now considered first-line therapy for tFL [16,17]. Because of the poor reported median survivals for patients with tFL, consolidative autologous stem cell transplant (auto-SCT) is often considered. Small cohorts, non-randomized design, and variable inclusion criteria and length of follow-up limit interpretation of reports of auto-SCT in tFL, and most of the published series do not reflect the now standard use of rituximab. Recent reports from transplant registries include larger patient cohorts, but they did not focus on initial therapy of patients diagnosed with transformed follicular lymphoma [18,19]. Consequently, the benefit and optimal timing of auto-SCT fort FL in the current immunotherapy era is not established.
We conducted a retrospective analysis of patients with tFL who were treated at our institution over a 15 year period to evaluate the impact of auto-SCT on survival. We limited our study population to patients with FL that transformed to DLBCL. Both patients with histologic transformation and those with clinical transformation who did not undergo biopsy were included, as previous reports indicate that these patients have similar survival [9]. Eighty-six percent of patients received rituximab as part of the initial therapy for tFL.
Methods
Study design
Patients treated for tFL between Jan 1, 1994 and March 31, 2009 was identified through a search of institutional clinical and surgical pathology databases. We performed a retrospective review of the medical records if patients had a pathologic diagnosis of both FL and DLBCL according to the 2008 WHO Classification definitions [20]. If biopsy results were not available at suspected transformation, we used criteria previously adopted by other authors to define clinical transformation, including a sudden rise in LDH, rapid localized nodal growth, new extra nodal sites of disease, hypocalcaemia, or the development of B symptoms [9,10]. Patients were excluded if no clinical information was available or if they were lost to follow-up prior to receiving therapy for tFL (n=7).
We grouped patients into 5 categories for analysis: 1) an initial biopsy demonstrated FL and biopsy from a later date demonstrated DLBCL; 2) initial biopsies had evidence of both FL and DLBCL; 3) initial biopsy demonstrated DLBCL, and subsequent biopsy demonstrated FL (in these cases, date of transformation was defined as date of DLBCL diagnosis); 4) DLBCL occurred in the CNS; 5) biopsy proven FL and clinical evidence of transformation as defined above.
For descriptive purposes, chemotherapy at the time of tFL diagnosis was divided into four groups: 1) CHOP; 2) R-CHOP; 3) platinum-based regimens; 4) other chemotherapy. Rituximab use, both for FL and at the time of transformation was also recorded. Response was determined using standard criteria and was documented by PET in 43 patients (41%) [21].
Statistical analysis
Progression free survival (PFS) was calculated from the date of DLBCL diagnosis (date of transformation) to the date of documented lymphoma progression, death or date the patient was last documented to be alive and free of progression. OS was calculated from the date of diagnosis of DLBCL until last contact or death. For patients who were lost to follow-up, PFS was calculated from date of last contact. Date of death, if available, was obtained from the Social Security Death Index database and used in OS calculations; otherwise date of last contact was used.
Categorical variables were compared by the chi-square or Fisher's exact test as appropriate and continuous variables were compared by the Kruskal-Wallis test. The survival probability was estimated using the Kaplan-Meier method and compared between treatment groups with the log-rank test. A Cox proportional regression model was used to evaluate factors which may be associated with OS and PFS after transformation. All statistical tests were 2-sided and statistical significance was indicated at p value <0.05. The statistical package SAS 9.3 was used for all the statistical analysis (SAS Institute Inc., Cary, NC). This study was approved by the institutional review board at Washington University in Saint Louis.
Results
Patient characteristics
Categorical variables were compared by the chi-square or Fisher's exact test as appropriate and continuous variables were compared by the Kruskal-Wallis test. The survival probability was estimated using the Kaplan-Meier method and compared between treatment groups with the log-rank test. A Cox proportional regression model was used to evaluate factors which may be associated with OS and PFS after transformation. All statistical tests were 2-sided and statistical significance was indicated at p value <0.05. The statistical package SAS 9.3 was used for all the statistical analysis (SAS Institute Inc., Cary, NC). This study was approved by the institutional review board at Washington University in Saint Louis.
All Patients
No transplant
Auto-SCT
n= 105
n= 81
n= 24
Demographics:
Median age, years (SD)
60 (11)
62(12)
55 (9)
Race, white, n (%)
96 (91)
73 (90)
23 (96)
Male, n (%)
53 (50)
43 (53)
10 (42)
IPI scorea, n (%)
0 or 1
21 (20)
11(14)
10 (42)
2
29 (28)
25 (31)
2 (8)
3
23 (22)
18 (22)
7 (29)
4 or 5
6 (5)
5 (6)
1 (4)
Unknown
26 (25)
22 (27)
4 (17)
Prior therapies, n (%)
0
59 (56)
50 (62)
9 (38)
1
22 (21)
14 (17)
8 (33)
2 or more
24 (23)
17 (21)
7 (29)
Prior rituximab exposure
32 (30)
21 (26)
11 (46)
Diagnostic criteria, n (%)
Group 1
58 (55)
39 (48)
19 (79)
Group 2
32 (30)
27 (33)
5 (21)
Group 3
8 (8)
8 (10)
0
Group 4
2 (2)
2 (2)
0
Group 5
5 (5)
5 (6)
0
aat the time of DLBCL diagnosis; auto-SCT: Autologous Stem Cell Transplant; SD: Standard Deviation; IPI: International Prognostic Index; FL: Follicular Lymphoma; DLBCL: Diffuse Large B Cell Lymphoma "Unknown" is excluded from the analysis in Fisher's exact test.
Table 1: Demographics.
Compared with the transplanted group, patients treated with chemotherapy alone were older (median age 62 versus 55, P=0.003) and had higher IPI scores (P=0.01). Fifty-five percent of the study population was not treated for FL prior to transformation. Fewer patients who underwent auto-SCT were previously untreated (38% versus62% of those receiving chemotherapy alone), but this difference was not statistically significant (P= 0.09). Thirty-two of 50 previously treated patients (64%) received rituximab prior to transformation. One patient in the non-transplanted group underwent an auto-SCT for refractory FL one year prior to transformation.
Five patients who did not receive transplant had a clinical diagnosis of transformation. The diagnosis of clinical tFL was made based on rapid localized nodal growth with focal necrosis on CT scan in two patients, one in association with hypocalcaemia and elevated LDH. The remaining three patients were diagnosed based on new extra nodal disease in the spleen, one with associated hypocalcaemia.
Treatment
At transformation 72% of patients received CHOP (n=7) or R-CHOP (n= 69). Twenty-two percent (n = 23) received platinumbased therapies such as ESHAP (topside, methylprednisolone, high-dose cytarabine and cisplatin) or ICE (ifosfamide, carboplatin, etoposide), with or without rituximab. Per institutional practice, platinum-based therapies were commonly used for patients who had previously received anthracyclines. Six patients received other chemotherapeutic regimens either because of CNS involvement or physician choice. Eighty-six percent of patients received rituximab as part of their initial therapy for tFL (n= 90), including 88% of the auto-SCT group and 85% of those treated with chemotherapy alone.
The most common preparative regimens for auto-SCT were BEAM (carmustine, topside, cytarabine and melphalan; 75%), and BEAC (carmustine, etoposide, cytarabine and Cyclophosphamide; 17%). Two patients underwent total body irradiation combined with Cyclophosphamide and etoposide.
Outcomes
With a median follow-up of 44 months, the 2-year and 5-year OS for all patients were 78% and 65%, respectively. After initial chemotherapy for tFL, 60 patients (57%) achieved CR, 31 (30%) PR, and 12 (11%) had PD. Two patients died prior to completion of initial chemotherapy.
Twenty-four patients underwent auto-SCT in first remission of tFL. Transplantation was performed a median of 5.4 months from the date of transformation (range 4.1 to 9.2 months), with 50% of patients in PR and 50% in CR at time of transplant. Following auto-SCT, 19 patients were in CR (79%), 2 (8%) in PR, and 3 (13%) had PD. There were no early treatment related deaths in the auto-SCT group. PFS was 54% at 2 years and 42% at 5 years, and OS was 88% at 2 years and 74% at 5 years.
Eighty-one patients received chemotherapy without auto-SCT. Of these, 82% responded to initial chemotherapy, with 48 (59%) CR and 19 (23%) PR. PFS was 57% at 2 years and 30% at 5 years and OS was 75% at 2 years and 63% at 5 years. There was no significant difference in survival among patients who underwent auto-SCT in first remission versus those who were treated with chemotherapy alone (Figures 1,2). OS and PFS of patients with clinical transformation did not differ from those with biopsy-proven transformation (OS, P=0.58; PFS, P=0.12).
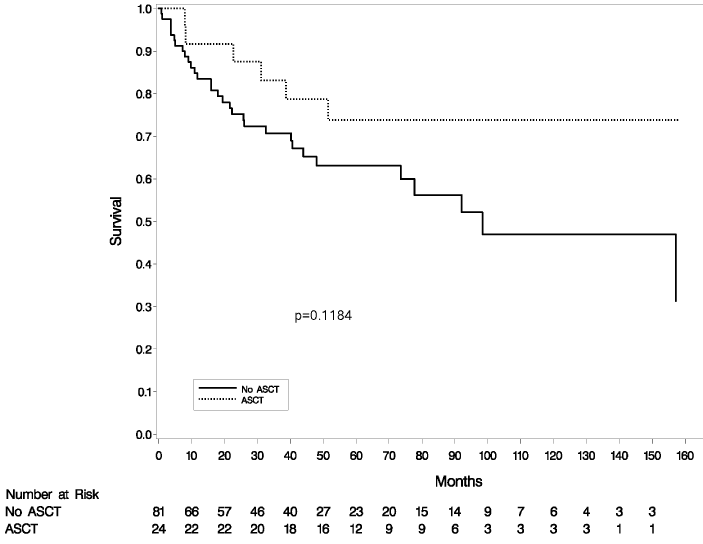
Figure 1: Overall survival, autologous stem cell transplant (ASCT) versus no ASCT (p=0.12).
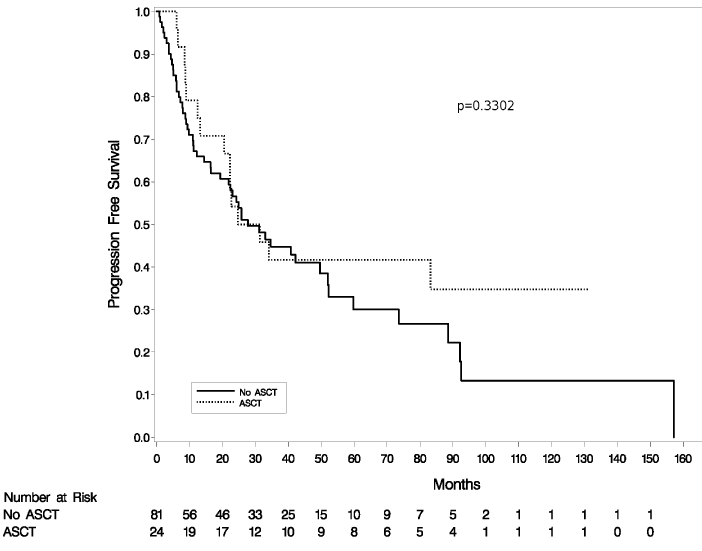
Figure 2: Progression free survival,autologous stem cell transplant (ASCT) versus no ASCT, (p=0.33).
Because many previous tFL studies excluded patients in whom diagnosis of FL and DLBCL occurred less than 6 months apart, or in whom diagnosis of DLBCL preceded FL diagnosis, we isolated these patients for analysis. There was no statistical difference in the outcome of these patients and those who had transformation > 6 months after the diagnosis of follicular lymphoma. Among 32 patients with evidence of both FL and DLBCL at initial diagnosis, 27 patients received chemotherapy alone (78% RCHOP; 10% CHOP; 6% platinum-based; 6% other). Respectively, 2 and 5 year OS rates were 84% and 74%, and 2 and 5 year PFS rates were 63% and 35%. Five patients with concurrently diagnosed follicular lymphoma and DLBCL received auto-SCT in first remission with 80% PFS and OS at 2 years.
To investigate whether certain subsets of patients benefit from auto-SCT, we stratified patients based on IPI score (0-1, 2, 3, and 4-5) and number of prior therapies (0, 1, and 2 or more). There was no significant difference in OS or PFS for auto-SCT versus no auto-SCT in any of the IPI strata. 56% of all patients were chemotherapy naïve at transformation. Of these, 9 underwent auto-SCT and 50 received chemotherapy alone. There was no difference in survival between these two groups (OS, P= 0.60; PFS, P= 0.45).
Factors influencing survival in tFL
In univariate analysis, age less than 60, and IPI score at time of transformation had a significant impact on OS but not PFS. Lack of chemotherapy exposure prior to transformation was associated with improved OS and PFS therapy (OS, P=0.007; PFS,P= 0.03). Among those with complete IPI data, an IPI score of 2 or greater (median survival 73 months) predicted a worse OS compared to those with IPI scores of 0 or 1, in whom median survival was not reached (P=0.02). In multivariate analysis, age greater than 60 (P=0.03), was the only significant predictors of poor OS, though prior chemotherapy exposure approached significance with p=0.06.
Relapse
Forty-eight patients relapsed after achieving remission, and 29 had a repeat biopsy performed. Biopsies revealed DLBCL in 45%, both FL and DLBCL in 7%, and isolated FL in 48%. Two patients with FL relapse subsequently developed recurrent DLBCL. Fourteen patients underwent auto-SCT for lymphoma relapse (seven for recurrent DLBCL; three for recurrent FL; and four who were not biopsied) at a median 31 months from transformation. Nine of these patients (64% of those who underwent salvage auto-SCT) are alive and in CR a median of 9 years from transformation and 2.8 years from transplant.
Discussion
Consolidative auto-SCT is considered for many patients with tFL because of historically poor outcomes with standard chemotherapy. Over the last decade, large randomized trials have supported the addition of rituximab to chemotherapy for both FL and DLBCL. A retrospective report suggests that rituximab also improves survival in patients with tFL [16]. We undertook a retrospective analysis to evaluate outcomes in 105 patients with DLBCL transformed from an underlying FL, focusing on a comparison between those who received auto-SCT in first remission and those who did not. Ninetytwo percent of patients (n= 97) in our series received rituximab either for FL or as initial therapy for tFL, and 86% had rituximab as part of their initial therapy for tFL.
We report higher OS rates than many previously published reports, with 78% of patients alive at 2 years and 65% alive at 5 years. There are several likely causes for the relatively good outcomes. Most importantly, studies published before the current immunotherapy era did not reflect the now standard practice of adding rituximab to the chemotherapy regimen. Additionally, our cohort included a high proportion of patients with factors that have been associated with improved prognosis. Almost half had IPI scores = 2and 56% were chemotherapy-naive at transformation. Finally, our definition of transformation enriched our cohort with patients who may be more likely to respond to therapy. Unlike some series which required a minimum of six months between FL and DLBCL diagnosis [12,22,23] we included patients with an initial diagnosis of DLBCL and FL and patients in whom DLBCL diagnosis preceded FL diagnosis. Early transformation has been associated with a better prognosis [11].
Studies investigating auto-SCT fort FL are summarized in Table 2 [24]. Reported the only prospective phase II study that evaluated the use of auto-SCT for tFL. Unfortunately, neither this nor we gian study was designed prior to the widespread use of rituximab in that country and did not meet the accrual goal. All patients had relapsed after treatment for their indolent lymphoma and had at least stage II disease. Thirty-four of 47 patients (64%) achieved CR or PR, and 30 underwent auto-SCT, with a 5 year OS of 47%. Only 10% had prior rituximab exposure.
Study
# patient
Med age (y)
Rituximab use
PFS
OS
Eide
30
55
11%
32% (5y)
47% (5y)
Hamadani
24
56
62%
33% (5y)
52% (5y)
Ban-Hoefen
18
58
100%
59% (2y)
82% (2y)
Williams
50
45
0%
30% (5y)
51% (5y)
Villa
97
55
100%
55% (5y)
65% (5y)
Wirk
108
56
28%
35% (5y)
50% (5y)
Current study
24
55
88%
42% (5y)
74% (5y)
Table 1: Published reports of auto-SCT for tFL.
Two recent registry reports provide the largest retrospective analyses of transplant outcomes in t-FL. Villa et al. reported a modest improvement in OS among 97 patients with tFL treated with auto- SCT (65%) versus 53 patients treated with rituximab containing regimen (61%) [18]. This large, retrospective, multicenter Canadian trial included patients with transformation to any high grade histology, and, in contrast to the current study, the authors did not distinguish between patients who received auto-SCT in first remission (approximately 50%) versus those who were transplanted later in their disease course. A report from the Center for International Blood and Marrow Transplant Research (CIBMTR) also included a mixed population of patients with tFL [19]. Among the 108 patients included in their series who underwent auto-SCT, 61% had received 3 or more prior therapies, and only 28% were treated with rituximab. In this population, PFS was 35% at 5 years.
Unlike other studies, ours focuses on patients receiving their first therapy for tFL, with 86% treated with rituximab-containing regimens at transformation. We did not detect a statistically significant difference in OS or PFS between patients treated with atuo-SCT in first remission of tFL compared to those who did not undergo transplant. In patients with both FL and DLBCL at initial diagnosis, consolidative auto-SCT can provide durable PFS, but the small number of these patients in our series prevents a significant comparison of atuo-SCT versus immune chemotherapy alone. Further studies are needed to determine the best approach in this subset of patients.
Interpretation of our results is limited by the inconsistent definition of tFL in the literature, the fact that patients were not prospectively assigned to their treatment group, and that patients who underwent auto-SCT had a younger median age, documented chemo sensitive disease and lower median IPI scores We observed higher PFS and OS rates in both treatment groups as compared to many historic studies, consistent with recent reports of improved survival in the rituximab era. Our results do not support consolidative auto- SCT for all patients with tFL in first remission, although it remains a reasonable option for selected patients, such as those tFL patients with significant previous chemotherapy.
Acknowledgement
This work was supported by funding from the Mentors in Medicine program, Department of Internal Medicine, Washington University School of Medicine. All authors contributed to the study design and data analysis, and all approved the manuscript. Jackson performed the data collection and wrote the manuscript.
References
- Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005; 23: 8447-8452.
- Giné E, Montoto S, Bosch F, Arenillas L, Mercadal S, Villamor N, et al. The Follicular Lymphoma International Prognostic Index (FLIPI) and the histological subtype are the most important factors to predict histological transformation in follicular lymphoma. Annals of oncology: official journal of the European Society for Medical Oncology / ESMO. 2006; 17: 1539-1545.
- Hiddemann W, Kneba M, Dreyling M, Schmitz N, Lengfelder E, Schmits R, et al. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005; 106: 3725-3732.
- Robert Marcus, Kevin Imrie, Philippe Solal-Celigny, John V Catalano, Anna Dmoszynska, Joao C. Raposo, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008; 26: 4579-4586.
- Montoto S, Fitzgibbon J. Transformation of indolent B-cell lymphomas. J Clin Oncol. 2011; 29: 1827-1834.
- Acker B, Hoppe RT, Colby TV, Cox RS, Kaplan HS, Rosenberg SA. Histologic conversion in the non-Hodgkin's lymphomas. J Clin Oncol. 1983; 1: 11-16.
- Hubbard SM, Chabner BA, DeVita VT Jr, Simon R, Berard CW, Jones RB, et al. Histologic progression in non-Hodgkin's lymphoma. Blood. 1982; 59: 258-264.
- Oviatt DL, Cousar JB, Collins RD, Flexner JM, Stein RS. Malignant lymphomas of follicular center cell origin in humans. V. Incidence, clinical features, and prognostic implications of transformation of small cleaved cell nodular lymphoma. Cancer. 1984; 53: 1109-1114.
- Al-Tourah AJ, Gill KK, Chhanabhai M, Hoskins PJ, Klasa RJ, Savage KJ, et al. Population-based analysis of incidence and outcome of transformed non-Hodgkin's lymphoma. J Clin Oncol. 2008; 26: 5165-5169.
- Bastion Y, Brice P, Haioun C, Sonet A, Salles G, Marolleau JP, et al. Intensive therapy with peripheral blood progenitor cell transplantation in 60 patients with poor-prognosis follicular lymphoma. Blood. 1995; 86: 3257-3262.
- Ghesquières H, Berger F, Felman P, Callet-Bauchu E, Bryon PA, Traverse-Glehen A, et al. Clinicopathologic characteristics and outcome of diffuse large B-cell lymphomas presenting with an associated low-grade component at diagnosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006; 24: 5234-5241.
- Micallef IN, Remstein ED, Ansell SM, Colgan JP, Inwards DJ, Johnston PB, et al. The International Prognostic Index predicts outcome after histological transformation of low-grade non-Hodgkin lymphoma. Leukemia & lymphoma. 2006; 47: 1794-1799.
- Montoto S, Davies AJ, Matthews J, Calaminici M, Norton AJ, Amess J, et al. Risk and clinical implications of transformation of follicular lymphoma to diffuse large B-cell lymphoma. J Clin Oncol. 2007; 25: 2426-2433.
- Morley NJ, Evans LS, Goepel J, Hancock BW. Transformed follicular lymphoma: the 25-year experience of a UK provincial lymphoma treatment centre. Oncol Rep. 2008; 20: 953-956.
- Yuen AR, Kamel OW, Halpern J, Horning SJ. Long-term survival after histologic transformation of low-grade follicular lymphoma. J Clin Oncol. 1995; 13: 1726-1733.
- Al-Tourah AJ, et al. Addition of Rituximab to CHOP Chemotherapy Significantly Improves Survival of Patients with Transformed Lymphoma. ASH Annual Meeting Abstracts. 2007; 110, 790.
- Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010; 116: 2040-2045.
- Villa D, Crump M, Panzarella T, Savage KJ, Toze CL, Stewart DA, et al. Autologous and allogeneic stem-cell transplantation for transformed follicular lymphoma: a report of the Canadian blood and marrow transplant group. J Clin Oncol. 2013; 31: 1164-1171.
- Wirk B, Fenske TS, Hamadani M, Zhang MJ, Hu ZH, Akpek G, et al. Outcomes of hematopoietic cell transplantation for diffuse large B cell lymphoma transformed from follicular lymphoma. Biol Blood Marrow Transplant. 2014; 20: 951-959.
- Swerdlow SH, CE, Harris NL, Jaffe ES, Pilert SA, Stein H, et al (editors). (2008) WHO Classification of Tumours of Haematopoietic and Lymphoid Systems. IARC, Lyon.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007; 25: 579-586.
- Ban-Hoefen M, Kelly JL, Bernstein SH, Liesveld J, Constine L, Becker M, et al. High-dose therapy and autologous stem cell transplant for transformed non-Hodgkin lymphoma in the rituximab era. Leuk Lymphoma. 2012; 53: 830-835.
- Hamadani M, Benson DM Jr, Lin TS, Porcu P, Blum KA, Devine SM. High-dose therapy and autologous stem cell transplantation for follicular lymphoma undergoing transformation to diffuse large B-cell lymphoma. Eur J Haematol. 2008; 81: 425-431.
- Eide MB, Lauritzsen GF, Kvalheim G, Kolstad A, Fagerli UM, Maisenhölder M, et al. High dose chemotherapy with autologous stem cell support for patients with histologically transformed B-cell non-Hodgkin lymphomas. A Norwegian multi centre phase II study. Br J Haematol. 2011; 152: 600-610.