
Case Report
Ann Hematol Oncol. 2014;1(1): 1003.
Male Choriocarcinoma with Pulmonary and Liver Metastases, Choriocarcinoma Syndrome, and Brain Metastasis: a Case Report and Review of the Literature
Gardner F, Wu K and Tan WW*
Mayo Clinic College of Medicine, USA
*Corresponding author: Winston Tan, Mayo Clinic College of Medicine, 4500 San Pablo Road, Jacksonville, FL 32224,USA
Received: August 14, 2014; Accepted: August 29, 2014; Published: September 01, 2014
This is a case of a 24-year old male who initially presented to a local hospital complaining of diffuse back pain that was ongoing for a few months. He did present several times to a walk-in clinic for his back pain and was treated with non-steroidal anti-inflammatory drugs with no improvement in his symptoms. He then developed a 20-pound weight loss, nausea and vomiting with dehydration which prompted his visit to a local ER. CT of the chest, abdomen, and pelvis was performed which showed a questionable retroperitoneal mass in addition to multiple lung nodules. The patient then presented to our hospital for further management.
He was admitted to the hospital for dehydration with electrolyte abnormalities which was treated with IV fluids and replenishment of electrolytes. Despite hydration therapy, the patient looked moribund. CT scan confirmed multiple nodular lesions involving all lobes of the lungs in addition to multiple nodular enhancing lesions in both lobes of the liver. He also had retroperitoneal adenopathy with a conglomerate of left retroperitoneum and retroperitoneal lymph nodes measuring 5.8 x 6.4 cm. His β-hCG was 434,500 with LDH of 592. Testicular ultrasound showed a 1.7 mass in the left testicle. There was no right testicular mass. Baseline MRI of the brain with and without contrast was negative for any intracranial metastases.
He then underwent fine needle aspiration of his liver lesion. The pathology showed malignant epithelioid cells which were positive for cytokeratin AE1/AE3 and negative for c-kit, CD30, and CD45. PAS was also positive in a few cells. OCT3/4 was negative. Given the history of a testicular mass, elevated HCG, the pathology was consistent with a malignant unclassified germ cell tumor.
β-hCG Levels (IU/L)
At diagnosis
619,000
One month after treatment
5,300
Two months after initial treatment
140
Four months after initial treatment
8
At recurrence
20
Prior to tandem transplant
23
Immediately after tandem transplant
0.8
Two years after tandem transplant
<0.6
Table 1: Trend of β-hCG levels at the time of diagnosis, after initial chemotherapy treatment, and after tandem transplant.
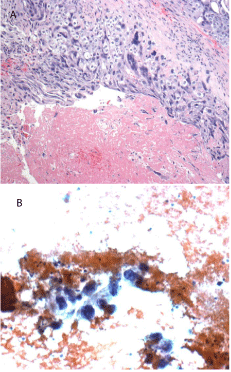
Figure 1A: H&E stain showing cytotrophoblasts and synctiotrophoblasts
consistent with choriocarcinoma of the testis (high power magnification).
Figure 1B: FNA of a liver lesion showed rare choriocarcinoma cells consistent
with metastatic disease.
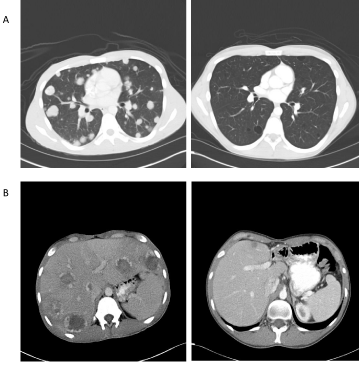
Figure 2: CT scans of pulmonary (A) and liver metastases (B) at the time of diagnosis and two years after completion of high-dose chemotherapy and tandem transplant.
The patient subsequently underwent a radical left orchiectomy. Pathology showed a large necrotic mixed germ cell tumor that was predominantly choriocarcinoma (90%) with a minor (10%) teratomatous component. Intratubular germ cell neoplasia was also present. However, his post-operative course was complicated by hemoptysis requiring support in the intensive care unit. At that time, he was unable to move without any significant pain and was not able to eat except sips of water. Pulmonary hemorrhage was confirmed by CT angiogram of the chest. Overall, his presentation was concerning for choriocarcinoma syndrome. He subsequently underwent thoracic aortography and bronchial angiography with prophylactic embolization of the right bronchial arteries.
As the patient had metastases to the liver as well as β-hCG >50,000, he was considered "poor risk". He was started on chemotherapy with VIP (etoposide, ifosapimide, and cisplatin) given concerns for bleomycin-induced lung injury. He received a total of four cycles of VIP-chemotherapy with Neulasta support. Clinically, he had significant improvement in his symptoms, going from ECOG performance status of 4 to ECOG of 1. His β-hCG also decreased from a high of 740,000 down to 1.4.
Then, during routine surveillance, the patient's β-hCG had increased to 20. Repeat staging evaluation including CT chest, abdomen, and pelvis showed decrease in size of the pulmonary nodules and liver lesions compared to previous examination with increase patchy ground glass changes in the right middle lobe concerning for acute inflammatory changes. A repeat MRI of the brain with and without contrast showed a new right frontal enhancing metastasis. There were also new punctuate foci of gradient echo hypointensity in the right parietal, right occipital lobe, and the right cerebellum concerning for early hemorrhagic metastases.
The patient was referred to Indiana University where he underwent 2- courses of high-dose chemotherapy followed by tandem stem cell transplantation. After transplantation, his tumor markers all normalized. His pulmonary metastases had nearly resolved. However, a repeat MRI of his brain revealed a smaller, but persistent abnormality. He then underwent surgical resection of his brain metastasis.
After his recovery from brain surgery, the patient then had a postchemotherapy retroperitoneal lymph node dissection with resection of a liver lesion. The pathology showed a viable teratoma in his left periaortic region with significant fibrosis. There was also viable teratoma in his inteaortocaval lymph node. Three of 6 lymph nodes were involved with tumor. The liver lesion showed only fibrotic tissue. Repeat brain MRI showed only postoperative changes but no evidence of residual disease.
Following this aggressive treatment, the patient was started on prophylactic etoposide at 50mg/m2/day in two divided doses every 21 days of a 28-day cycle for 3 months. During that time, he was monitored with chest x-ray every 2 months and CT of the chest, abdomen and pelvis every 4 months for the first two years. Two years after his initial diagnosis, the patient remains in remission.
Introduction
Testicular choriocarcinoma is a very rare, aggressive type of germ cell tumor (GCT) accounting for less than 5% of all germ cell tumors [1]. Advanced choriocarcinoma is often associated with extremely high levels of β-hCG levels as well as high-volume tumors with early dissemination [2]. Like choriocarcinoma in women, choriocarcinoma in men is often associated with hemorrhagic complications, which can sometimes be fatal [3,4].
In 1984, Logothetis first described choriocarcinoma syndrome, characterized by hemorrhage at the sites of metastases containing high-volume choriocarcinoma elements with significantly elevated β-hCG levels. Acute pulmonary hemorrhage is the most common manifestation, though hemorrhage any at site of metastasis can develop [2]. Clinical manifestations which suggest pulmonary involvement by metastatic choriocarcinoma include hemoptysis, cough, and tumor emboli but nontraumatic hemothorax is rare [5].
Hemorrhagic complications from choriocarcinoma can occur in two settings: immediately after the start of chemotherapy and/or in those patients with rapidly progressive disease [3]. Treatment of choriocarcinoma syndrome includes intensive supportive measures as well as in some cases surgical intervention including thoroactomy or lobectomy [3,6]. In some cases, selective arterial embolization can be performed to help manage internal bleeding [7-9].
Nevertheless, despite aggressive measures including intensive medical and surgical management, patients who develop hemothorax or hemorrhagic shock usually do not survive [6]. Prompt recognition of choriocarcinoma syndrome with intense and rapid intervention of hemorrhagic complications with different modalities of therapy may lead to patient's survival so that he may benefit from curative therapy as was the case with our patient.
The International Germ Cell Collaborative Group (IGCCG) classified nonseminomatous germ cell tumor (NSGCT) as good-, intermediate-, and poor-risk disease with 5-year overall survival rates of 91%, 79%, and 48% respectively [10]. For all "poor risk" patients, bleomycin, etoposide, and cisplatin (BEP) remains the standard of care, though etoposide, ifosfamide, and cisplatin (VIP) is equivalent [11]. As our patient developed choriocarcinoma syndrome with acute pulmonary hemorrhage, bleomycin was excluded due to concerns of bleomycin-induced lung injury. Our patient had a significant response to therapy, as marked by his rapidly declining β-hCG to normal levels, shrinkage of his pulmonary and liver lesions, and resolution of his symptoms with dramatic improvement of his performance status from ECOG of 4 to 1. Unfortunately, a few months, later our patient developed brain metastasis.
Despite the improved cures for patients with metastatic NSGCT, those who developed brain metastases generally have a poor outcome. Approximately 2% to 3% of patients with disseminated testicular cancer will developed brain metastases with a reported median survival time of 3 to 4 months [12,13]. However, long-term survival can be achieved using cranial irradiation or cisplatin-based chemotherapy or a combination of both modalities [14]. Indeed, in one of the largest case series done, Bokemeyer et al. found that approximately 25% of patients with brain metastases from testicular cancer achieved long-term survival with brain irradiation and aggressive cisplatin-based chemotherapy [13].
The best long-term outcome for patients with refractory and relapsed germ cell cancer usually involves a multidisciplinary approach including salvage therapy with high-dose chemotherapy (HDCT) with autologous hematopoietic stem-cell transplantation to rescue the bone marrow from the myeloablative effects of chemotherapy [15- 18]. Indeed, HDCT has become the standard treatment for patients with relapsed or refractory germ cell tumors (GCTs) either as firstline or subsequent salvage therapy [18]. Resection of residual tumors has also been shown to be an integral part of any salvage strategy in patients with GCTs because of the higher rates of viable cancer or teratoma [19].
In a retrospective study done at Indiana University, Einhorn and colleagues found that metastatic testicular tumors are potentially curable by high-dose chemotherapy and stem cell rescue, even if the regimen is a third-line or later therapy or if the patient has platinumrefractory disease [18]. Of the 184 patients reviewed, 116 (63%) had complete remission of disease without relapse during a median followup of 48 months. This regimen consisted of the administration of two consecutive courses of high-dose chemotherapy with carboplatin and etoposide followed by the infusion of peripheral blood stem cells for hematopoietic stem-cell rescue. Those patients who had a complete or partial remission received maintenance therapy with three cycles of oral etoposide, as was the case with our patient [18].
In conclusion, we present the clinical course of a patient with testicular cancer consisting mainly of choriocarcinoma whose course was complicated by choriocarcinoma syndrome. The patient was initially treated with chemotherapy despite a poor performance status. Yet, although he had a good initial response to therapy, within a short time interval, he developed recurrent disease with brain metastasis. Aggressive management of his recurrence with multimodalities of therapies including high-dose chemotherapy with tandem transplant and aggressive metastasectomies led to cure of his disease. Aggressive management of testicular cancer, even in the worse cases such as those with brain metastasis can potentially cure some patients.
References
- Schmoll HJ, Souchon R, Krege S, Albers P, Beyer J, Kollmannsberger C, et al. European consensus on diagnosis and treatment of germ cell cancer: a report of the European Germ Cell Cancer Consensus Group (EGCCCG). Ann Oncol. 2004; 15: 1377-1399.
- Logothetis CJ, Samuels ML, Selig DE, Ogden S, Dexeus F, Swanson D, et al. Cyclic chemotherapy with cyclophosphamide, doxorubicin, and cisplatin plus vinblastine and bleomycin in advanced germinal tumors. Results with 100 patients. Am J Med. 1986; 81: 219-228.
- Motzer RJ, Bosl GJ. Hemorrhage: a complication of metastatic testicular choriocarcinoma. Urology. 1987; 30: 119-122.
- Johnson DE, Appelt G, Samuels ML, Luna M. Metastases from testicular carcinoma. Study of 78 autopsied cases. Urology. 1976; 8: 234-239.
- Sudduth CD, Strange C, Campbell BA, Sahn SA. Metastatic choriocarcinoma of the lung presenting as hemothorax. Chest. 1991; 99: 527-528.
- Tatokoro M, Kawakami S, Sakura M, Kobayashi T, Kihara K, Akamatsu H, et al. Successful management of life-threatening choriocarcinoma syndrome with rupture of pulmonary metastatic foci causing hemorrhagic shock. Int J Urol. 2008; 15: 263-264.
- Kawai K, Takaoka E, Naoi M, Mori K, Minami M, Shimazui T, et al. A case of metastatic testicular cancer complicated by tumour lysis syndrome and choriocarcinoma syndrome. Jpn J Clin Oncol. 2006; 36: 665-667.
- Lok CA, Reekers JA, Westermann AM, Van der Velden J. Embolization for hemorrhage of liver metastases from choriocarcinoma. Gynecol Oncol. 2005; 98: 506-509.
- Shen K, Yang X, Song H, Liu W, Zhang C. Selective arterial embolization in the management of internal bleeding caused by trophoblastic diseases. Chin Med J (Engl). 1996; 109: 151-156.
- [No authors listed]. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997; 15: 594-603.
- van Dijk MR, Steyerberg EW, Habbema JD. Survival of non-seminomatous germ cell cancer patients according to the IGCC classification: An update based on meta-analysis. Eur J Cancer. 2006; 42: 820-826.
- Williams SD, Einhorn LH. Brain metastases in disseminated germinal neoplasms: incidence and clinical course. Cancer. 1979; 44: 1514-1516.
- Bokemeyer C, Nowak P, Haupt A, Metzner B, Köhne H, Hartmann JT, et al. Treatment of brain metastases in patients with testicular cancer. J Clin Oncol. 1997; 15: 1449-1454.
- Logothetis CJ, Samuels ML, Trindade A. The management of brain metastases in germ cell tumors. Cancer. 1982; 49: 12-18.
- Bhatia S, Abonour R, Porcu P, Seshadri R, Nichols CR, Cornetta K, et al. High-dose chemotherapy as initial salvage chemotherapy in patients with relapsed testicular cancer. J Clin Oncol. 2000; 18: 3346-3351.
- Motzer RJ, Sheinfeld J, Mazumdar M, Bains M, Mariani T, Bacik J, et al. Paclitaxel, ifosfamide, and cisplatin second-line therapy for patients with relapsed testicular germ cell cancer. J Clin Oncol. 2000; 18: 2413-2418.
- Murphy BR, Breeden ES, Donohue JP, Messemer J, Walsh W, Roth BJ, et al. Surgical salvage of chemorefractory germ cell tumors. J Clin Oncol. 1993; 11: 324-329.
- Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R, et al. High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med. 2007; 357: 340-348.
- Rick O, Bokemeyer C, Weinknecht S, Schirren J, Pottek T, Hartmann JT, et al. Residual tumor resection after high-dose chemotherapy in patients with relapsed or refractory germ cell cancer. J Clin Oncol. 2004; 22: 3713-3719.