
Case Report
Ann Hematol Oncol. 2014;1(2): 1006.
Treatment of Contralateral Axillary Metastases: Palliative Vs Curative Dilemma
Dayyat A1, Sbaity E2, Mula-Hussain L3, Youssef B4, Kaouk I4, Amin Hassan A
1Department of Radiation Oncology, King Hussein Cancer Center, Jordan
2Department of Surgery, American University of Beirut Medical Center, Lebanon
3Sulaimania Radiation Oncology Center, Sulaimania, Iraq
4Department of Radiation Oncology, American University of Beirut, Lebanon
5King Faisal Specialist Hospital and Research Center, Riyadh, Kingdom of Saudi Arabia
6Augusta-Victoria Hospital Cancer Care Center, Jerusalem
7"Sapienza" University of Rome, Italy
8Assiut University, Egypt
9King Abdul Aziz Medical Center, Saudi Arabia
10Department of Surgery, Memorial Sloan Kettering Cancer Center, USA
11Department of Medicine, Memorial Sloan Kettering Cancer Center, New York, NY, USA
12Department of Medicine, Weill Cornell Medical College, New York, NY, USA
13Department of Internal Medicine, American University of Beirut Medical Center, Lebanon
*Corresponding author: Nagi El-Saghir, Professor and Director of Breast Center of Excellence, NK Basile Cancer Institute and Division of Hematology Oncology, Department of Internal Medicine, American University of Beirut Medical Center, P.O. Box: 11-0236, Riad El Solh 1107 2020, Beirut, Lebanon
Received: August 02, 2014; Accepted: September 29,2014; Published: October 01, 2014
Abstract
Contralateral axillary metastasis that develops following treatment of breast cancer is a rare but challenging clinical scenario. We report the case that was discussed at the Inaugural Memorial Sloan Kettering Cancer Center and American University of Beirut Medical Center Joint Course in Oncologic Sciences catered for young oncologists. The course took place in Beirut, Lebanon between June 23 and 28, 2013. The case was presented by the trainees, discussed and built into a case report. The patient is a 63 year old woman who was treated for locally advanced breast cancer with neo-adjuvant chemotherapy followed by left Modified Radical Mastectomy (MRM), radiotherapy and hormonal therapy and who presented 4 years later with isolated contralateral axilla metastases. This was treated as stage IV disease with second-line hormonal therapy. A year later, the disease was still limited to the axilla and decision was made to approach the patient with a curative intent. She underwent right axillary dissection followed by chemotherapy and radiotherapy. Fourteen months after her last treatment, the patient has no evidence of disease. Atypical axillary metastases from contralateral breast cancer could represent a regional disease that is potentially curative. Due to the potential for long-term survival, an active and curative intent treatment of patients with similar presentation may be appropriate.
Keywords: Breast cancer; Contralateral axillary node metastases; Lymphatic drainage
Introduction
Breast cancer is the most common cancer in women. The estimated new cases and mortality among US women in 2012 were 226,870 and 39,510 respectively [1]. Contralateral Axillary lymph node Metastases (CAM) following prior treatment of breast cancer is uncommon with a reported incidence of 1.9% - 5% [2-4]. The contralateral involvement of the axilla in a woman with a current diagnosis or history of having been treated for cancer in the opposite breast represents a diagnostic dilemma. There are three possible clinical scenarios to be considered. First, it could be the manifestation of systemic disease by hematogenous spread from the original breast tumor. Second, it could be regional metastases from an occult new ipsilateral breast primary. Third, it could still represent regional metastases from the contralateral breast cancer due to transport of breast cancer to the contralateral axilla by means of chest wall deep lymphatic fascialplexi or across the midline via dermal lymphatics [5]. In all cases, the management of such patient's is perplexing since it is not clear whether they should be treated in a palliative or curative intent. Traditional wisdom supports considering such patients as stage IV and treats them with systemic therapy. Nonetheless, there are numerous published reports which narrate more favorable outcomes with more aggressive therapy [2-4,6,7,13]. In either case, patients require careful clinical and radiological evaluation to exclude new occult breast cancer, or in rare occasions, metastases from a tumor outside the breast. Pathological confirmation and correlation with the initial tissue is essential. Possible treatment options for patients with CAM include surgery, systemic therapy and radiotherapy.
We report the case that was discussed at the Inaugural Memorial Sloan Kettering Cancer Center and American University of Beirut Medical Center Joint Course in Oncologic Sciences that catered foryoung oncologists. The course took place in Beirut, Lebanon between June 23 and 28, 2013. The case was presented by the trainees, discussed and built into a case report. All trainees and faculty who participated in this specific case study were included as co-authors.
Case Presentation
A 63 year old postmenopausal woman was treated at our center for a left breast cancer. She initially presented with an ulcerating locallyadvanced left breast carcinoma and ipsilateral axillary palpable matted adenopathy. She underwent staging body CT scan and bone scan and those did not reveal any evidence of distance metastases. Accordingly, her clinical stage was cT4bN2M0. Immunohistochemical stains on formalin fixed paraffin embedded sections showed positivity for Estrogen and Progesterone Receptors (ER/PR) while HER-2/neu was negative. The patient received neo adjuvant chemotherapy in the form of four cycles of doxorubicin and cyclophosphamide, followed by four cycles of docetaxel. She achieved very good clinical response to chemotherapy with a reduction in the size of the breast mass as well as the axillary adenopathy and disappearance of the skin ulceration. After that, she underwent left modified radical mastectomy that revealed a 2.5cm grade II/III residual invasive ductal carcinoma in the upper outer quadrant with free surgical margins and presence of lympho vascular invasion. Seven out of sixteen lymph nodes showed metastatic deposits (ypT2N2). She received external beam radiotherapy as 42.9 Grays (Gy) in 13 Fractions (Fx) to the left chest wall and supraclavicular fosse followed by a cone-down boost of 14 Gy in 7 Fx to the mastectomy scar. Hormonal treatment consisted of Letrozole 2.5 mg daily.
The patient was subsequently followed up every 3-4 months with clinical examinations as well as annual right mammograms. Four years after the initial diagnosis she developed lymph node enlargement in the contralateral axilla. Mammogram, ultrasound and Magnetic Resonance Imaging (MRI) of the right breast showed multiple enlarged malignant looking axillary adenopathy with no radiologic evidence of a primary tumor in the breast (Figure1). Clinically, there was no evidence of disease in the site of her originally treated left breast. A biopsy from the right axilla showed metastatic carcinoma consistent with breast primary and the hormone receptor assays for ER/PR were positive and HER-2/neu was negative. Staging with a computed tomographic torso scan and bone scan revealed no distant sites of metastasis. The patient was considered to have metastatic left breast cancer and was treated in a palliative fashion by switching the hormonal therapy to exemestane. After a year, the right axillary lymph nodes exhibited mild disease progression with no evidence of disease elsewhere in her body. A right axillary lymph node clearance was carried out and revealed metastatic mammary carcinoma in 22 out of 23 lymph nodes with extra capsular extension and lymphovascular invasion (Figure 2). This was followed with adjuvant chemotherapy as four cycles of cyclophosphamide and docetaxel. Hormonal therapy, in the form of tamoxifen 20mg daily, was resumed upon completion of chemotherapy. In addition, radiotherapy as 50Gy/25Fx wasdelivered to the right breast and supraclavicular fossa with careful attention not to be overlapping with the previous radiation fields. She tolerated the whole treatment fairly well without complications. Her post treatment routine surveillance CT scans of the body did not show any evidence of relapse at 14 months following surgery.
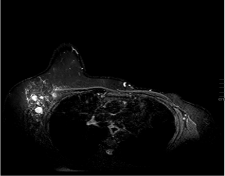
Figure 1: MRI of the right breast showing abnormal right axillary LN with negative findings in the right breast.
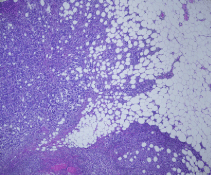
Figure 2: Metastatic mammary carcinoma with extracapsular extension.
Discussion
Lymphatic spread to the contralateral axilla
The regional lymphatic drainage of the breast is to the ipsilateral axillary, internal mammary and supraclavicular lymph nodes [8]. In a study from the Netherlands, 700 patients with clinically node-negative breast cancer underwent lymphatic mapping with preoperative lymphoscintigraphy followed by Sentinel Lymph Node Biopsy (SNB) [9]. The radiotracer mapped to the axilla, internal mammary chain and the supraclavicular fossa and none of the included patients exhibited contralateral axillary drainage.
Interruption of the lymphatic channels by surgery, or radiotherapy or blockage of the lymphatics by travelling tumor cells, may result in development of altered drainage [3]. Perre et al. reported different patterns of lymphatic drainage of the breast after treatment of breast cancer by surgery and radiotherapy [10]. Crossing over to the contralateral nodes was observed seven times in six patients and when this is explained by altered lymphatic drainage, then these nodes can be regarded as part of the regional lymphatic basin. Van der Ploeg et al [11] conducted a study of 115 patients analyzing the lymphatic drainage patterns after previous treatment of breast cancer.
Characteristics of contralateral axillary metastases (CAM)
CAM can either occur at time of diagnosis of breast cancer (synchronous CAM) or months or years later (metachronous CAM). The majority of published data report more aggressive features of breast cancer when associated with CAM [2,3]. Morcos et. al. [3] provided the largest series, and they retrospectively analyzed 21 patients with CAM and compared them to 401 breast cancer patients without CAM. In this study, the authors demonstrated that patients with CAM had significantly worse histopathological features such as higher tumor grade, more advanced initial tumor stages, Lympho Vascular Invasion (LVI), ER receptor negativity and HER-2/neu over expression. Similarly, our patient exhibited poor pathological features in the initial tumor (cT4N2, grade II and positive LVI). These findings are consistent with the poor pathological features reported in Daoud's series [2] (Table 1). While nearly all patients in Morcos and Daoud series demonstrated locally advanced disease on initial workup, all Huston's patients had stage I or II. Kulik et al [13] reported the characteristics and outcome of 13 CAM patients from single institution. They observed that dermal involvement at the primary site, initially or at time of recurrence, was associated with 77% ofthe cases. Daoud also hypothesized that the closer the tumor to the midline the more likely crossing it becomes. However, Huston and Morcos did not find such an association reinforcing the difficulty in predicting CAM.
Study
Year
Patients no.
Mean age (range)
locally advanced stage initially
LVI positivity
Hormone receptor positivity
HER-2/neu overexpression
Synchronous/Metachronous CAM
Daoud
1998
6
48 (28-60)
100%
-
-
-
3/3
Huston
2007
7
52 (35-65)
0%
80%
29%
80%
1/6
Morcos
2011
21
50 (29-71)
90%
81%
48%
42%
10/11
Kiluk
2014
13
53 (26-72)
38%
-
62%
15%
10/3
Kiluk
2014
13
53 (26-72)
38%
-
62%
15%
10/3
Table 1: Patients and Tumor Characteristics in Patients with CAM.
Management of CAM
The management of patients with CAM represents a clinicaldilemma. Current evidence is based upon small retrospective series and case reports with diverse patients characteristics [2-4, 6,7,13]. Therefore, we recommend individualized treatment taking intoaccount the primary tumor characteristics and the tumor free interval. The initial step should be to identify the origin of the LN disease by a core biopsy, or an excision biopsy. After securing a diagnosis of breast carcinoma in the nodes, it is important to exclude associated occult breast cancer by careful examination utilizing available imaging modalities especially with MRI scan. If the metastatic work-up is negative and CAM is the only site of the disease then eradicating the tumor by surgery is probably the most appropriate option in an attempt to achieve a longer disease free survival [4]. Systemic treatment afterwards with or without radiotherapy may provide additional benefit (Table 2). While only 4 of the 21 patients in Morcos series had axillary dissection, 6 of the seven patients in Huston's series had dissection or removal of the involved nodes [3,4]. The longest progression free intervals in these two series are 45 and 35 months, respectively. The 10 patients in Kiluk's series, who were not known to have any other metastatic deposits, underwent axillary clearance for CAM eradication. Three of these were reported to be free of disease with a follow up period of 8.2 years for one of the cases [13]. Interestingly, these three patients had metachronous presentation of contralateral disease suggesting a favorable outcome compared to the synchronous presentations from the same series. However, there was no such association in Morcos and Huston series. Mastectomy is generally not indicated unless high suspicion of an occult ipsilateral primary is present or for risk reduction in hereditary cancers [3]. Devitt et al [14] reported the presence of occult breast cancer in two out of six patients who had ipsilateral mastectomies for CAM. Breslow [15] reported six women with metastatic carcinoma in contralateral axillary nodes some time after a radical mastectomy. With no other evidence of tumor, a second radical mastectomy was performed, and new primary tumor was found in four of the six women.
Author
Year
No. of patients
Median Follow up
Surgery for CAM, (n)
Chemotherapy, (n)
Hormonal therapy,
(n)
Radiotherapy,(n)
outcome
Daoud et. al. [2]
1998
6
-
ALND,(4)
Yes, (5)
None
Yes,(5)
2 NED
4 AWD
Huston et. al.[4]
2007
7
35 months
ALND, (5) excision of +LN, (1)
Yes, (7)
Yes, (1)
None
2 NED
3 AWD
2 DOD
Kinoshia et. al. [6]
2010
1
36 months
ALND
Yes
Yes
Yes
NED
Morcos et. al. [3]
2011
21
27 months
ALND, (4)
Yes (21)
Yes, (7)
None
5 NED
12 AWD
4 DOD
C. Zhou et. al. [7]
2013
1
27 months
ALND
Yes
Yes
Yes
AWD
Kiluk et. al.
2014
13
38 months
ALND, (10)
Yes, (13)
Yes, (5)
Yes, (5)
3 NED
1 AWD
10 DOD
Table 2: Treatment of CAM and Oncologic Outcome.
Systemic treatment is another approach for CAM treatmentChemotherapy or hormonal therapy (if hormone responsive) might be considered in some cases. Morcos argued that locally advanced tumors with poor pathological features are more likely to develop overt systemic metastases later on in the course of their disease. In his opinion, systemic treatment (with hormonal therapy being the first choice if hormone responsive) alone would provide acceptable control sparing them unnecessary surgeries.
Our patient had a locally advanced tumor initially and sustained a disease free interval of more than three years. After CAM diagnosis, hormonal treatment alone could provide good tumor control for almost a year. Until the time of writing this paper, she maintained a disease free interval of almost fourteen months after axillary dissection. As this is still considered stage IV disease, chemotherapy was utilized with the intent of improving relapse-free survival. Although it was not utilized in Morcos and Huston's series, we offered radiotherapy to our patient. This would probably add to the local control, besides treating possible occult ipsilateral breast primary. The NCCN recommends adding radiotherapy after axillary dissection for occult breast cancer following the treatment guidelines of stage II breast cancer [16]. We also can hypothesize that radiotherapy could eradicate the microscopic disease in the dermal lymphatics that could have spread to the contralateral axilla from the original treated side.
Conclusion
The development of CAM after previous treatment of breast cancer is a challenging clinical situation. Thinking of it as a metastatic disease and treating patients in a palliative fashion is too simplistic. Altered lymphatics by previous surgery and radiotherapy or even tumor cells in the lymphatic channels may play a role in the development of aberrant pathways leading to CAM. Although compelling evidence is lacking, treatment of CAM should be of curative intent as patients might achieve a longer disease free interval with a multimodality approach that includes axillary dissection, radiation therapy, and appropriate systemic therapy.
Source of Funding
This work was presented at the Memorial Sloan Kettering Cancer Center and the American University of Beirut Inaugural Joint Course in Oncologic Sciences, 23rd- 28th June, 2013, Beirut-Lebanon. This conference was supported by endowment gift of Mrs. Mamdouha El- Sayed Bobst and the Bobst Foundation.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012; 62: 10-29.
- Daoud J, Meziou M, Kharrat M, Sellami D, Boudawara T, Frikha M. [Contralateral axillary lymph node metastasis of cancer of the breast]. Bull Cancer. 1998; 85: 713-715.
- Morcos B, Jaradat I, El-Ghanem M. Characteristics of and therapeutic options for contralateral axillary lymph node metastasis in breast cancer. Eur J Surg Oncol. 2011; 37: 418-421.
- Huston TL, Pressman PI, Moore A, Vahdat L, Hoda SA, Kato M, et al. The presentation of contralateral axillary lymph node metastases from breast carcinoma: a clinical management dilemma. Breast J. 2007; 13: 158-164.
- Jaffer S, Goldfarb AB, Gold JE, Szporn A, Bleiweiss IJ. Contralateral axillary lymph node metastasis as the first evidence of locally recurrent breast carcinoma. Cancer. 1995; 75: 2875-2878.
- Kinoshita S, Hirano A, Kobayashi S, Komine K, Kyoda S, Takeyama H, et al. Metachronous secondary primary occult breast cancer initially presenting with metastases to the contralateral axillary lymph nodes: report of a case. Breast Cancer. 2010; 17: 71-74.
- Zhou C, Richir MC, Leenders MW, Langenhorst BL, Knol HP, Schreurs WH. Contralateral axillary lymph node metastases at the time of primary breast cancer diagnosis: curative or palliative intent? Case Rep Surg. 2013; 2013: 389013.
- Green FL, Page DL, Fleming ID, Balch Charles M, Fritz April G. AJCC cancer staging manual. 6th edn. New York, Springer-Verlag. 2002.
- Estourgie SH, Nieweg OE, Olmos RA, Rutgers EJ, Kroon BB. Lymphatic drainage patterns from the breast. Ann Surg. 2004; 239: 232-237.
- Perre CI, Hoefnagel CA, Kroon BB, Zoetmulder FA, Rutgers EJ. Altered lymphatic drainage after lymphadenectomy or radiotherapy of the axilla in patients with breast cancer. Br J Surg. 1996; 83: 1258.
- van der Ploeg IM, Oldenburg HS, Rutgers EJ, Baas-Vrancken Peeters MJ, Kroon BB, Valdés Olmos RA, et al. Lymphatic drainage patterns from the treated breast. Ann Surg Oncol. 2010; 17: 1069-1075.
- Allweis TM, Parson B, Klein M, Sklair-Levy M, Maly B, Rivkind A, et al. Breast cancer draining to bilateral axillary sentinel lymph nodes. Surgery. 2003; 134: 506-508.
- Kiluk JV, Prowler V, Lee MC, Khakpour N, Laronga C, Cox CE. Contralateral axillary nodal involvement from invasive breast cancer. Breast. 2014; 23: 291-294.
- Devitt JE, Michalchuk AW. Significance of contralateral axillary metastases in carcinoma of the breast. Can J Surg. 1969; 12: 178-180.
- Breslow A. Occult carcinoma of second breast following mastectomy. JAMA. 1973; 226: 1000-1001.
- NCCN clinical practice guidelines 2013.