
Case Report
Ann Hematol Oncol. 2014;1(2): 1007.
A Patient with Metastatic Clear Cell Renal Carcinoma with Long Term Survival Spanning the Era of Pre-Targeted to Molecular Targeted Treatment
John A Copland1, Jerome Marcus2, Kevin Wu3, Joseph G. Cernigliaro4 and Winston W. Tan5*
1Department of Cancer Biology, Mayo Clinic Comprehensive Cancer Center, USA
2Spring Drive, Wichita KS
3Department of Pathology and Laboratory Medicine, Mayo Clinic Comprehensive Cancer Center, USA
4Department of Diagnostic Radiology, Mayo Clinic Comprehensive Cancer Center, USA
5Division of Hematology & Oncology, Mayo Clinic, USA
*Corresponding author: Winston Tan, Division of Hematology & Oncology, Mayo Clinic Comprehensive Cancer Center, 4500 San Pablo Road, Jacksonville, Florida, 32224, USA
Received: August 23, 2014; Accepted: October 13, 2014; Published: October 15, 2014
Abstract
Clear Cell Renal Cell Carcinoma (ccRCC) is the most common subtype of kidney cancer, and has the highest risk of developing metastatic disease. Long term survival with durable response remains uncommon despite numerous FDA approved targeted therapies. We report a patient with metastatic ccRCC who survived for 14 years using a number of evolving approaches spanning the pre-targeted era of immunotherapy to present day molecular targeted treatment. These multimodality approaches that included cytoreductive surgery has led to long term survival and a continued high quality of life.
Keywords: Interleukin 2; Clear cell renal cell carcinoma; Molecular targeted therapy; Quality of life; Pulmonary metastasis
Introduction
Renal Cell Carcinoma (RCC) is one of the most common solid tumors, responsible for over 13,000 deaths annually with an incidence rate of over 63,000 per year in the United States [1]. The clear cell variant (ccRCC) is the most common subtype of RCC, accounting for an estimated 80% of all patients [2]. The prognosis for patients diagnosed with early stage disease is good, with patients demonstrating approximately 70% cure rate [3]. Regrettably, up to 30% of early stage cases of ccRCC treated surgically will relapse with metastatic disease due to the presence of undetectable micrometastases [4]. In addition, 20-30% of all ccRCC patients present with advanced or metastatic disease upon initial diagnosis [5].
Metastatic ccRCC renders a bleak prognosis, with an estimated 5 year overall survival of less than 10% due to lack of remedial therapies that produce significant disease regression or attenuation of disease progression [6]. Drug resistance is a hallmark of ccRCC and is thought to be a culmination of several intrinsic and acquired tumorigenic properties linked to cancer cell heterogeneity, including a lack of known molecular factors which can be targeted pharmacologically [7-11]. ccRCC does not respond to chemotherapy and radiation therapy, and even with targeted treatments drug resistance develops rapidly [8,12]. Interleukin-2 (IL-2) is the only drug approved today by the Food and Drug Administration (FDA) that provides long-term durable response in metastatic ccRCC patients [13]. However, the majority of patients are not able to take this therapy due to multiple co-morbidities and multiple life-threatening toxicities [14]. Only a small subset of patients demonstrate a complete response (7%) but this subset cannot be accurately identified prior to therapy [15]. It is also apparent that ccRCC tumor cells demonstrate a disposition for increased migratory capacity, likely a major contributing factor to the development of tumor metastasis and disease relapse.
In this case report, we present the strategies that have led to a continued high quality of life for over 14 years in a patient with metastatic ccRCC to multiple anatomical sites. Evolving treatment strategies will be shared in chronological order of management of recurring and new sites of metastases.
Case Report
In September 2000, a very active, athletic 58 year-old male in otherwise excellent health began to have progressive weight loss of 18 pounds, night sweats, anemia, fatigue, indigestion and back pain over a four month period. In January of 2001, Computed Tomography (CT) scan revealed a 12.2 x 12.2 mass in the left upper quadrant originating from the upper pole of the left kidney. There was a caudal and anterior displacement of the left kidney by the mass.
A Nephrectomy of the left kidney was performed January 21, 2001anda diagnosis of ccRCC was made. Pathology revealed an 11 cm tumor that had grown into the vena cava. No lymph node involvement was identified but a 1 cm spot on the lung was noted and initially diagnosed as a granuloma. Gross description showed a massively necrotic tumor, nearly in its entirety, and occupying greater than 90% of the kidney. The tumor extends through the surface and into the perinephric fat. The tumor had the following characteristics: extensive necrosis, marked lymphocytic response and Fuhrman nuclear grade IV. Surgical vascular margins are free of tumor. Tumor extends through the renal capsule and into the adrenal gland. Adjacent kidney parenchyma showed extensive angiolymphatic invasion. Following the surgery, the indigestion and back pain resolved immediately.
In April 2001, a repeat CT of the chest showed multiple noncalcified lung nodules with the largest (1 cm) within the posterior segment of the right upper lobe. Due to the short recurrence interval, a clinical diagnosis of metastatic kidney cancer to the lungs was made. At this visit, a discussion occurred related to treatment options. Three modalities of interleukin 2 (IL-2) administrations were discussed. The option chosen was intermediate subcutaneous treatment of IL-2 self-administered at home.
A therapeutic choice - Immunotherapy
On April 30, 2001, self-injection of s.c. IL-2 was started with eighteen million units (mu) on Monday through Friday of the first week followed by nine mu units on Monday and Tuesday and then eighteen mu units on Wednesday, Thursday and Friday of the next five weeks to make a six-week cycle. Three weeks off between cycles was followed by a CT scan to check progress each time. A total of thirteen cycles was repeated over two and a half years (390 injections). For the last five cycles, the time between cycles was increased to four weeks, then five, then six weeks. The toxicities that the patient experienced included fever (101 – 103C, 2 hours after injection), chills for about 30 – 60 minutes and fatigue. Follow up CT scan (8/21/2001), revealed that lung metastases had shrunk. The greatest reduction in visible metastases was observed between 2nd and 3rd cycles of IL-2. CT of the pelvis was normal. In December, 2001, CT scans demonstrated continued shrinkage of lung metastases. By March 2002 all lesions were stable. In July 2002, a PET scan found no hypermetabolic focus in the lung and patient was declared to be in complete remission.
It should be noted that the same week that IL-2 was started (April 2001), allergy shots(1.2 cc each of ragweed mix 400 PNU/ml mold mix 1-10 w/v and mixed mites 500 AU/ml) were reinitiated at 0.5 cc weekly dose. Initially, allergy shots began as a teenager and continued into the late 30’s.
Discovery of new metastasis and advent of molecular targeted treatment
In June 2007, an abdominal CT scan showed an 8x7x10 cm mass in the peri-colonic area (Figure 1A). In July 2007, a surgical resection was performed that partially removed the colon and gall bladder. Histology confirmed metastatic ccRCC (Figure 1B). In July 23, 2007, IL-2 s.c. therapy was repeated for 3.5 months (11/9/2007) with no CT scan response. During this time, several new small subcentimeter nodules were detected in the lungs and were followed with CT scans.
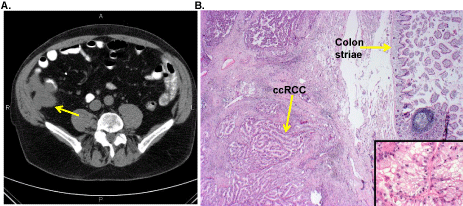
Figure 1: ccRCC metastasis to the colon. A. CT scan demonstrating invasion of ccRCC into the colonic lining (yellow arrow) and B. pathologic confirmation of colonic invasion by ccRCC in the resected colon specimen. Bottom right panel insert is a 100x magnification of ccRCC metastasis.
In January 2008, a 1.3 x 1.9 cm lesion in the posterior mid superior right pubiswas detected (Figure 2A). Radiation to the right pubic bone (January, 2008) did not cause tumor regression. Zoledronic acid (3.5 mg i.v. every 3 months) was begun February 8, 2008 through November 3, 2008 for the right pubic bone to decrease the incidence of skeletal related events. Due to osteonecrosis of the jaw, the zoledronic acid was discontinued.
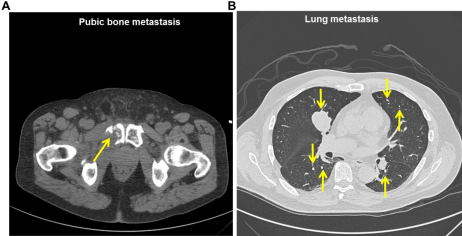
Figure 2: Pubic bone metastasis. A. CT scan of the pelvis revealing pubic bone metastasis measuring 33.3 x 28.6 mm (yellow arrow). B. Multiple bilateral lung metastasis were identified on a CT scan (yellow arrows).
By April 2008, fourteen metastatic lesions continued to increase in size in the lung (Figure 2B). Due to travel, temsirolimus (i.v.) was ruled out as a therapeutic option and sunitinib (50 mg; 4 weeks on/ two weeks off) was started on April 14, 2008 until August 7, 2009. Stable disease was observed after 2 cycles. On July 28, 2009, he became symptomatic and a cryoablation for the pubic bone metastasis was performed with no success. Additionally, tumor embolization of the largest artery feeding the tumor was performed on November 18, 2009. This procedure led to stable disease.
Follow up of lung metastasis in 2009, showed progression of disease and sunitinib was discontinued on August 4, 2009. Subsequently, everolimus (10 mg PO daily) treatment initiated on August 9, 2009. On September 11, 2009, significant reduction of 75% of the 14 lesions was shown by CT scan. CT scans on October 27, 2009 showed continued response in the lungs with progression in the hips. Bevacizumab was added to the regimen to target the tumors growing in the hip. Bevacizumab (800 mg i.v. every 2 weeks) was started on December 9, 2009 through August 13, 2010 which stabilized tumor growth. Due to tumor progression, everolimus and bevacizumab were discontinued and pazopanib (800 mg p.o. daily) was started in August, 2010 through August 2011.This treatment provided positive benefit for a year with partial response of lesions in the lung.
In November 2011, 5-fluorouracil (5FU) combined with gemcitabine was started due to tumor progression. Mixed tumor response of lung metastasis was noted. In June, 2012, drug treatment was halted even though disease was stable. The drug combination caused acute renal failure (10% of total capacity). Dialysis was discussed. A low potassium and phosphorous diet was begun in June, 2012. This alleviated the need for dialysis. In February, 2013, eight months after ceasing therapy, CT and MRI scans of lung and abdomen showed stable disease. Presently, the patient is off treatment and asymptomatic despite the lung and bone metastases. He continues to lead an active life.
Table 1: Sample Text.
Discussion
This case illustrates the breath of the transformation and evolution of therapy for metastatic ccRCC therapy. The initial years were focused on immunotherapy followed by the era of molecular targeted treatment and more recently the era of refining combinatorial therapy of metastatic ccRCC. This patient appears somewhat unique in that each of the administered therapies provided clinical benefit. For instance, s.c. IL-2 is thought to be inferior to high dose i.v. IL-2. However, in this patient, s.c. IL-2 led to long term durable complete remission for 2.5 years with excellent quality of life. Simultaneously, when IL-2 benefit was waning, novel molecular targeted treatments became available to patients diagnosed with metastatic ccRCC. Also at this time, studies identified patients with oligo-metastases from metastatic ccRCC benefited from resection of the metastases [16]. In this patient, an abdominal metastasis touching the colon was noted and subsequent resection led to long-term remission.
Because immunotherapy provided the survival benefit to this patient, there was an opportunity to administer novel targeted therapies as they became FDA approved and available for the treatment of metastatic ccRCC. Clinically, we now know that patients who have partial response to IL-2, typically benefit from other multiple sequential treatments with molecular targeted therapy [17,18]. However, this is not the case for most patients. In addition, the health care team and the patient thought that the allergy shots may have enhanced the response to immunotherapy. The patient continues weekly allergy shots.
A small percentage of physicians continue to treat patients with first line IL-2 because of its potential for durable long-term response [18]. However, due to its toxicity and low response rate, widespread use of this therapy is limited. More commonly, first line therapy includes Vascular Endothelial Growth Factor (VEGF) inhibitors such as sunitinib and pazopanib [19-21]. A recent study compared sunitinib and pazopanib demonstrating similar efficacy, however, pazopanib led to a better Quality of Life (QOL) in patients [21]. Although, bevacizumab and interferon are approved in first line setting, they are rarely used by clinicians due to significant toxicity and the need to administer the drugs parentally [22]. An mTOR inhibitor, temsirolimus, is approved as a first line therapy for poor risk metastatic ccRCC as defined by the Motzer criteria [23]. On tumor progression, the second line therapy of choice is the mTOR inhibitor, everolimus [24] or the VEGF inhibitor, axitinib [25]. The patient in this report responded to sunitinib for a year as well as everolimus and pazopanib. The subsequent proper sequencing of the drugs discussed continue to be an area of active investigation [26]. Interestingly, despite the subsequent aggressive progression of lung metastasis, this patient, in a sixth line setting, responded to an older chemotherapy combination of 5-fluorouracil and gemcitabine.
A last important point related to this case report is that the physicians treating this patient were cognizant of the goals of the patient’s desire to preserve quality of life. Consistent with this concept, the patient chose self-administration of s.c. IL-2 due to its lower incidence of toxicity and better quality of life while on treatment. Thus, through the combined efforts of the treating physicians and the patient, educated decisions were made leading to continued response and long-term quality of life. In summary, this report illustrates a case of long-term survival with multimodality approaches to the treatment of metastatic ccRCC as novel therapies evolved.
Funding Support
This work was funded in part from NIH/NCI grants R01CA104505 and R01CA104505-05S1,a generous gift from the David & Lois Stulberg Endowed Fund for Kidney Cancer Research, Mr. and Mrs. Ompal Chauhan Research Fund (JAC); Christian Haub Family Career Development Award in Cancer Research honoring Dr. Richard F. Emslander (WT).
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63: 11-30.
- Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005; 23: 2763-2771.
- Lam JS, Shvarts O, Pantuck AJ. Changing concepts in the surgical management of renal cell carcinoma. Eur Urol. 2004; 45: 692-705.
- Li G, Passebosc-Faure K, Lambert C, Gentil-Perret A, Blanc F, Oosterwijk E, et al. The expression of G250/mn/CA9 antigen by flow cytometry: its possible implication for detection of micrometastatic renal cancer cells. Clin Cancer Res. 2001; 7: 89-92.
- Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005; 353: 2477-2490.
- Haddad H, Rini BI. Current treatment considerations in metastatic renal cell carcinoma. Curr Treat Options Oncol. 2012; 13: 212-229.
- Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: memorial sloan-kettering cancer center experience. Clin Cancer Res. 2004; 10: 6302S-3S.
- Coppin C, Kollmannsberger C, Le L, Porzsolt F, Wilt TJ. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011; 108: 1556-1563.
- Von Roemeling CA, Radisky DC, Marlow LA, et al. Neuronal Pentraxin 2 is a regulator of clear cell renal cell carcinoma malignancy through activation of the AMPA-selective glutamate receptor 4. Cancer Research. 2014.
- Von Roemeling CA, Marlow LA, Radisky DC, Rohl A, Larsen HE, Wei J, et al. Functional genomics identifies novel genes essential for clear cell renal cell carcinoma tumor cell proliferation and migration. Oncotarget. 2014; 5: 5320-5334.
- von Roemeling CA, Marlow LA, Wei JJ, Cooper SJ, Caulfield TR, Wu K, et al. Stearoyl-CoA desaturase 1 is a novel molecular therapeutic target for clear cell renal cell carcinoma. Clin Cancer Res. 2013; 19: 2368-2380.
- Harshman LC, Xie W, Bjarnason GA, Knox JJ, MacKenzie M, Wood L, et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol. 2012; 13: 927-935.
- Belldegrun AS, Klatte T, Shuch B, LaRochelle JC, Miller DC, Said JW, et al. Cancer-specific survival outcomes among patients treated during the cytokine era of kidney cancer (1989-2005): a benchmark for emerging targeted cancer therapies. Cancer. 2008; 113: 2457-2463.
- Fisher RI, Rosenberg SA, Fyfe G. Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am. 2000; 6 Suppl 1: S55-57.
- Leibovich BC, Han KR, Bui MH, Pantuck AJ, Dorey FJ, Figlin RA, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003; 98: 2566-2575.
- Alt AL, Boorjian SA, Lohse CM, Costello BA, Leibovich BC, Blute ML. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer. 2011; 117: 2873-2882.
- Jonasch E, Futreal PA, Davis IJ, Bailey ST, Kim WY, Brugarolas J, et al. State of the science: an update on renal cell carcinoma. Mol Cancer Res. 2012; 10: 859-880.
- Birkhäuser FD, Pantuck AJ, Rampersaud EN, Wang X, Kroeger N, Pouliot F, et al. Salvage-targeted kidney cancer therapy in patients progressing on high-dose interleukin-2 immunotherapy: the UCLA experience. Cancer J. 2013; 19: 189-196.
- Bukowski RM. Critical appraisal of pazopanib as treatment for patients with advanced metastatic renal cell carcinoma. Cancer Manag Res. 2011; 3: 273-285.
- Motzer RJ, Hutson TE, Olsen MR, Hudes GR, Burke JM, Edenfield WJ, et al. Randomized phase II multicenter study of the efficacy and safety of sunitinib on the 4/2 versus continuous dosing schedule as first-line therapy of metastatic renal cell carcinoma. J Clin Oncol. 2012; 30: 1371-1377.
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013; 369: 722-731.
- Rini BI, Bellmunt J, Clancy J, Wang K, Niethammer AG, Hariharan S, et al. Randomized phase III trial of temsirolimus and bevacizumab versus interferon alfa and bevacizumab in metastatic renal cell carcinoma: INTORACT trial. J Clin Oncol. 2014; 32: 752-759.
- Mekhail TM, Abou-Jawde RM, Boumerhi G, Malhi S, Wood L, Elson P, et al. Validation and Extension of the Memorial Sloan-Kettering Prognostic Factors Model for Survival in Patients With Previously Untreated Metastatic Renal Cell Carcinoma. J Clin Oncol. 2005; 23: 832-841.
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008; 372: 449-456.
- Motzer RJ, Escudier B, Tomczak P, Hutson TE, Michaelson MD, Negrier S, et al. Axitinib vs sorafenib as second-line therapy for metastatic renal cell carcinoma (mRCC): results of phase 3 AXIS trial. J Clin Oncol. 2011; 29: s4503.
- Zustovich F, Lombardi G, Nicoletto O, Pastorelli D. Second-line therapy for refractory renal-cell carcinoma. Crit Rev Oncol Hematol. 2012; 83: 112-122.