
Research Article
Ann Hematol Oncol. 2014;1(3): 1012.
Colon Cancer Staging in Vulnerable Older Adults: Adherence to National Guidelines and Impact on Survival
Leal TB1,2, Holden T2, Cavalcante L2, Allen GO3, Schumacher JR3, Smith MA3, Weiss JM1,5, Neuman HB4 and LoConte NK1,2,3*
1University of Wisconsin Carbone Cancer Center, USA
2Department of Medicine of Hematology/Oncology, University of Wisconsin Section, USA
3University of Wisconsin Health Innovation Program, USA
4Department of Surgery, University of Wisconsin, USA
5Department of Medicine, University of Wisconsin, USA
*Corresponding author: LoConte NK, Department of Medicine and Carbone Cancer Center, University of Wisconsin, 600 Highland Ave, CSC K4/530, Madison, WI 53792, USA
Received: October 31, 2014; Accepted: November 26, 2014; Published: November 28, 2014
Abstract
Background: There is concern that elders are not adequately evaluated prior to colon cancer surgery. We sought to determine adherence with ACOVE-3 (Assessing Care of Vulnerable Elders) quality indicators for pre-operative staging prior to colectomy for colon cancer utilizing the Surveillance, Epidemiology and End Results (SEER)-Medicare linked database (1992-2005).
Methods: We determined the proportion of patients aged 75 and older who had preoperative staging prior to colectomy for colon adenocarcinoma. Preoperative staging was defined as abdominopelvic computed tomography or magnetic resonance imaging scan (SCAN) and colonoscopy or flexible sigmoidoscopy (SCOPE). Multivariate logistic regression identified predictors of adherence. Odds ratios were adjusted for comorbidity, socioeconomic status, and disease severity. The association of adherence to ACOVE-3 and survival was quantified.
Results: Of the 37,862 patients, the majority were 75-84 years, 28% of the patients were >85 years. Regarding preoperative staging in the 6-month interval prior to surgical resection, 8% had neither SCAN nor SCOPE, 6% had only SCAN, 43% had only SCOPE, and 43% had both SCAN and SCOPE. Compared to patients who were not staged, those evaluated with either SCOPE alone or SCAN plus SCOPE had lower odds of 3-year mortality. Patients who were staged with SCAN alone had an increased odds of death compared to those who had neither SCAN or SCOPE.
Conclusion: These data demonstrate that the majority of vulnerable elders with colon cancer did not receive appropriate preoperative staging prior to resection. The findings also confirm that adherence to ACOVE-3 guidelines is associated with improved long-term survival.
Keywords: ACOVE-3; Colon cancer; Quality indicators; Vulnerable elders; SEER-Medicare
Introduction
Colorectal cancer remains the second leading cause of cancerrelated death in the United States, despite the advances in the multidisciplinary treatment. Colon cancer is more common as one age, with age being one of the predominant risk factors for its development. By 2030, the segment of the population aged 80 and older is projected to reach 19.5 million and by 2050 this figure will reach 34 million or 8% of the total population. Thus, colon cancer, a disease of the elderly, is becoming an ever more significant population health concern [1]. The last two decades have seen an improvement in colon cancer survival, which has been attributed to better screening, staging, surgery, and systemic therapy [2]. However, significant disparities in colon cancer outcomes persist, especially for older adults. A study based on the California Cancer Registry demonstrated worse outcomes for octogenarians and nonagenarians with colorectal cancer in terms of morbidity, mortality, and readmission rates compared with younger patients [3]. There is limited information available about measuring the quality of medical care that is targeted to the needs of older patients receiving treatment for colon cancer. Measuring the quality of medical care for ill older adults is complex, because they tend to have multiple medical comorbidities, and there is substantial variation in goals of care [4]. The Assessing Care of Vulnerable Elders (ACOVE) project was created in 1998 to develop and apply quality indicators for the medical care of vulnerable older persons [4]. The project focuses on the 20-40% of community-dwelling older people who are at moderate to high risk of death or decline in Instrumental Activities of Daily Living (IADL) or Activities of Daily Living (ADL) over 2 years [5]. This group uses a disproportionate number of health care resources and is most susceptible to the effects of poor quality care. The ACOVE quality indicators are based on the Donabedian quality model [6], which focuses on processes of care. In 2007, the third phase of the project, ACOVE-3, introduced new indicators for a number of conditions including colon cancer. ACOVE-3 included two recommendations related to preoperative staging of older adults with colon cancer: 1) cross-sectional imaging of the abdomen and pelvis and 2) endoscopic evaluation of the entire colon [7]. Improved preoperative colon cancer staging in older adults has the potential to limit unnecessary surgery for those with metastatic cancer and improve outcomes for individuals with potentially curable disease. However, little is known about the frequency and patterns of colon cancer staging in vulnerable older adults. The association between adherences to ACOVE-3 proposed staging guidelines and long-term survival has not been studied using population-based data. In this study we addressed these knowledge gaps by analyzing the national Surveillance, Epidemiology and End Results (SEER)-Medicare linked database to describe patterns of colon cancer staging in older adults and their relationship to survival.
Methods
This study was prospectively reviewed by the University of Wisconsin-Madison Health Sciences Institutional Review Board and determined to be exempt under Code of Federal Regulations Title 45 Part 46.101(b).
Data sources
We obtained data from the SEER-Medicare linked database for patients diagnosed with colon cancer between 1992 and 2005. SEER-Medicare database combines Medicare administrative claims data and detailed clinical tumor registry data. It is one of the few population-based data resources available for the analysis of cancer care quality [8,9]. The SEER program of cancer registries collects information about patient demographics, tumor characteristics, first course of treatment, and survival for persons newly diagnosed with cancer. For people who are Medicare eligible, the SEER-Medicare database includes information on covered health care services, including hospital, physician, outpatient, home health, and hospice claims. The linkage of persons in the SEER database to their Medicare claims is performed by the National Cancer Institute (NCI) and the Centers for Medicare and Medicaid Services (CMS), with a linkage success rate of 93% [8,10]. SEER registries from 1992 to 2002 contain incident cancer diagnoses in the following cities, states, and regions: Los Angeles, San Francisco-Oakland, San Jose-Monterey, Greater California, Connecticut, Detroit, Atlanta, Rural Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, Seattle-Puget Sound, and Utah. In 2000, SEER regions included approximately 26% of the United States population [8].
Patients
All Medicare-enrolled patients aged 75 years and older diagnosed with primary colon adenocarcinoma in a SEER area from 1992 to 2005 were evaluated for inclusion in the study. Included patients had a diagnosis of American Joint Committee on Cancer (AJCC) stage I, II, III, or unstaged colon (SEER cancer site codes 18.0 -18.9, and 19.9) adenocarcinoma (SEER histology codes 8140-47, 8210-11, 8220-21, 8260-63, 8480-81, and 8490). Patients with mucinous cystadenocarcinoma (histology code 8470) were excluded because the natural history of this disease, which occurs in the appendix and is associated with pseudomyxoma peritonei, is different than other histologic subtypes of colon adenocarcinoma [11]. Patients with rectal cancer were also excluded because the surgical treatment of rectal canceris different from that of colon cancer, is often more technically challenging, and may be associated with a higher rate of complications. Patients were required to be continuously enrolled in parts A and B of fee-for-service Medicare for the 12 months preceding cancer diagnosis to ascertain comorbidity and for an additional 6 months after surgical discharge or until death, whichever came first, to enable tracking procedures. Patients enrolled in health maintenance organizations were excluded as their billing information could not be obtained from this database. All included patients underwent primary tumor resection, corresponding to International Classification of Diseases, ninth revision, Clinical Modification (ICD- 9-CM) procedure codes 45.7X (partial excision of large intestine) and 45.8X (total intra-abdominal colectomy). Patients were excluded if they did not undergo tumor resection within 6 months of diagnosis as it was felt that these patients either had rapidly progressive disease or comorbidities which dictated their ability to tolerate surgery. Patients were also excluded if they were diagnosed with another malignancy 1 year before or after the date of colon cancer diagnosis as this other cancer diagnosis was thought to possibly influence the treatments offered for their colon cancer. Patients were also excluded if their first diagnosis of colon cancer was made after death (i.e., on autopsy).
Outcome variable
The primary outcome of interest was preoperative staging in accordance to ACOVE-3 quality indicators. We defined adherence to the cross-sectional imaging indicator (SCAN) as receipt of an abdominopelvic computed tomography (CT: CPT-4 codes 74150, 74160, 74170, 72192, 72193, 72194) [12] or magnetic resonance imaging (MRI: CPT-4 codes 74181, 74185, 72196, 72198) [12] within 6 months prior to surgical resection. The ACOVE-3 indicator related to preoperative endoscopic evaluation of the colon (SCOPE) was defined as either colonoscopy (HCPCS codes G0105, G0121, 44388- 44389, 45378, 45380, 45382-45385 and ICD-9-CM 45.23, 45.25, 45.41-45.43, 48.36) [13] or flexible sigmoidoscopy (HCPCS codes G0104, 45330-45331, 45333, and 45338-45339, and ICD-9-CM 45.22, 45.24, 48.22, and 48.24) [13], within 6 months prior to surgery. Adherence to ACOVE-3 staging recommendations was categorized as no staging, SCAN only, SCOPE only, or both SCAN and SCOPE for each patient in the study. A secondary outcome measure, 3-year mortality, was created based on dates of death recorded in the SEER Patient Entitlement and Diagnosis Summary File (PEDSF) according to Social Security Administration data.
Predictor variables
Information on date of birth, gender, marital status, and race/ ethnicity was obtained from the SEER database. Census tract level median household income and median level of education were obtained from the PEDSF and used as proxies for patient socioeconomic status. Geographic region represented by SEER registry and rural/urban residence based on Rural/Urban Commuting Area Codes were also obtained from the PEDSF. To measure comorbidity, we used CMS Hierarchical Condition Categories (HCCs) [14] based on outpatient and inpatient diagnoses from the 12 months prior to colon cancer diagnosis. We also recorded the number of hospitalizations for each individual in the year prior to cancer diagnosis. In addition to the patient-related variables described above, we measured a variety of disease-related variables. American Joint Committee on Cancer (AJCC) stage and tumor grade was obtained from the SEER database. To allow adjustment for acuity of illness, we identified patients who presented with intestinal obstruction or perforation (ICD-9-CM diagnosis codes 560.89 and 560.9, respectively), and those who were emergently admitted prior to colectomy.
Statistical analysis
We determined adherence to preoperative SCAN and SCOPE prior to colectomy for colon cancer. We compared the frequency of patient-related (age, gender, race/ethnicity, census-tract based income and education, SEER registry, urban/rural residence, hospitalization in the year prior to colon cancer surgery, HCC comorbidity score) and disease-related (stage, grade, obstruction, perforation, emergent admission) variables in patients who did and did not have preoperative staging. We used logistic regression to analyze these data and determine adjusted ORs and 95% Confidence Intervals (CIs) of adherence to preoperative staging for different predictors, controlling for the other patient- and disease- related factors. Logistic regression was also used to quantify the association between staging patterns and 3-year mortality. Analyses were performed using Stata 13.1 software (Statacorp, College Station, Texas). All tests of significance were at the P < 0.05 level, and P values were 2-tailed.
Results
Patient characteristics
A total of 37,862 individuals met the inclusion criteria for the study and characteristics are summarized in Table 1. Of the 37,862 patients identified, 28% of the patients were >85 years, and the remainder were 75-84 years. Most patients were female (62%), Caucasian (87%), married or widowed (86%) and resided in a major metropolitan area 56% (see Table 1). The most frequent stage at diagnosis was stage II (44%), followed by stage III (29%), stage I (24%) and unstaged (4%). The majority of tumors was located in the right-side of the colon (58%) and had moderately-differentiated adenocarcinoma histology (66%). A significant proportion of admissions were coded as nonelective (60%) and the frequency of obstruction and perforation at presentation were 4% and 2%, respectively.
Characteristic
N (%)
Age
75-84
27,433 (72%)
>85
10,429 (28%)
Female gender
23,312 (62%)
Race/Ethnicity
White
33,099 (87%)
Black
2,074 (5%)
Other
2,689 (7%)
Marital status
Married
16,714 (44%)
Widowed
15,964 (42%)
Single, separated or divorced
3,889 (10%)
Unknown
1,295 (3%)
Median household income ($), mean (SD)
38,675 (17,238)
Less than 12 yr. education (%), mean (SD)
20 (12)
SEER registry
Connecticut
4,137 (11%)
Detroit
3,766 (10%)
Hawaii
592 (2%)
Iowa
5,010 (13%)
New Mexico
859 (2%)
Seattle
2,662 (7%)
Utah
1,058 (3%)
Atlanta & Rural Georgia
1,373 (4%)
Kentucky
1,745 (5%)
Louisiana
1,394 (4%)
New Jersey
4,127 (11%)
California
11,139 (29%)
Residence location
Major metropolitan area
21,224 (56%)
Metropolitan or urban
12,806 (34%)
Less Urban or Rural
3,830 (10%)
HCC comorbidity score, mean (SD)
2.0 (1.3)
Hospitalized in the year before surgery
10,986 (29%)
Cancer stage
I
8,980 (24%)
II
16,486 (44%)
III
10,964 (29%)
Unstaged
1,432 (4%)
Tumor grade
Well-differentiated
3,425 (9%)
Moderately differentiated
25,054 (66%)
Poorly differentiated or undifferentiated
7,769 (20%)
Unknown
1,614 (4%)
Admission Type
Emergent Admission
8,084 (21%)
Urgent Admission
22,729 (60%)
Others
7,049 (19%)
Colon Cancer Location
Right
21,995 (58%)
Transverse
3,825 (10%)
Left
3,225 (8%)
Sigmoid
8,195 (22%)
Other
595 (2%)
Intestinal obstruction on admission
1,526 (4%)
Intestinal perforation on admission
584 (2%)
Table 1: Characteristics of 37,862 Medicare beneficiaries aged 75 and older who underwent resection of colon cancer from 1992 to 2005 in the SEER-Medicare database (% indicates column percentage).
Preoperative staging
Of the 37,862 patients who underwent colon cancer resection, 7% had neither SCAN nor SCOPE, 6% had only SCAN, 43% had only SCOPE and 42% had both SCAN and SCOPE (Figure 1).
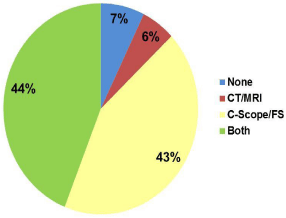
Figure 1: Frequency of adherence to ACOVE-3 preoperative staging
guidelines in 37,862 Medicare beneficiaries who underwent colectomy
for colon cancer. SCAN refers to abdominopelvic computed tomography
or magnetic resonance imaging scan; SCOPE, colonoscopy or flexible
sigmoidoscopy.
Predictors of adherence to ACOVE-3 staging guidelines are shown in Table 2. Patients with Asian/Pacific Islander or Hispanic race/ethnicity (other) had higher odds of adherence than whites (OR=1.33, 95% CI=1.21-1.45). We observed regional variation (as determined by SEER site) in odds of adherence to preoperative staging. However, rural or urban residence location was not significantly associated with adherence to preoperative staging (data not shown). A history of hospitalization in the year prior to surgery was a significant predictor of adherence (OR=1.34, 95% CI=1.28- 1.41). Of the disease-related variables, patients that had an urgent admission had higher odds of adherence compared to patient who were admitted emergently (OR=1.18, 95% CI=1.12-1.25). Patients with poorly-differentiated grade had higher odds of adherence than patients with well-differentiated histology (OR=1.22, 95% CI=1.12-1.34).
Variable
Adjusted odds ratio
95% CI
Race/ethnicity
Caucasian
Reference
Black
1.13
1.02-1.25
Other
1.33
1.21-1.45
Marital status
Married
Reference
Widowed
1.03
0.98-1.09
Single, separated or divorced
1.05
0.97-1.13
Unknown
0.94
0.83-1.06
Hospitalization in the previous year
1.35
1.28-1.41
No
Reference
Yes
1.35
1.29-1.42
Stage
Stage I
Reference
Stage II
1.2
1.13-1.27
Stage III
1.2
1.12-1.27
Unstaged
1.23
1.09-1.39
Tumor grade
Well differentiated
Reference
Mod. differentiated
1.10
1.02-1.19
Poorly or undiff.
1.22
1.12-1.34
Unknown
0.83
0.73-0.95
Acuity of admission
Emergent
Reference
Urgent
1.18
1.12-1.25
Others
0.85
0.79-0.91
Table 2: Predictors of adherence to ACOVE-3 preoperative staging guidelines in 37, 862 Medicare beneficiaries who underwent colectomy for colon cancer. Odds Ratios (ORs) and Confidence Intervals (CIs) are adjusted for age, gender, SEER registry, rural-urban residence location, tumor location, census-tract median household income, census-tract education, and all of the variables listed in the table.
Long-term survival
We next examined the association between patterns of preoperative staging and 3-year mortality. Compared to patients who were not evaluated with either modality, those who underwent both SCAN and SCOPE had decreased odds of death (OR=0.75, 95% CI=0.69-0.82). Patients who had SCOPE alone also had decreased odds of 3-year mortality (OR=0.71, 95% CI=0.65-0.78). However, the group evaluated with SCAN alone had increased odds of mortality at 3 years (OR 1.22, 95% CI=1.08-1.38) (Table 3).
Preoperative Staging
Crude OR
95% CI
Adjusted OR
95% CI
None
Reference
Reference
SCAN only
1.37
1.22-1.54
1.22
1.08-1.38
SCOPE only
0.56
0.52-0.61
0.71
0.65-0.78
SCAN plus SCOPE
0.64
0.59-0.69
0.75
0.69-0.82
Table 3: Association of preoperative staging studies and three-year mortality in 37,862 Medicare beneficiaries who underwent colectomy for colon cancer. SCAN refers to abdominopelvic computed tomography or magnetic resonance imaging scan; SCOPE, colonoscopy or flexible sigmoidoscopy. Adjusted Odds Ratios (ORs) and Confidence Intervals (CIs) are adjusted for age, gender, race, marital status, SEER registry, rural-urban residence location, hospitalization in the previous year, tumor stage, tumor location, tumor grade, admission type, census-tract median household income, and census-tract education; OR, odds ratio; CI, confidence interval.
Discussion
In this study we analyzed population-level SEER-Medicare data to describe patterns of preoperative staging in older adults with colon cancer. We found that only 44% of patients aged 75 and older were evaluated with both cross-sectional imaging such as abdominal computed tomography or magnetic resonance imaging (SCAN) and endoscopic examination of the colon (SCOPE), the two quality indicators related to colon cancer staging in ACOVE-3. We identified a number of patient and disease-related predictors of adherence to staging guidelines. Finally, we confirmed the association between adherence to staging recommendations and long-term survival in this population of vulnerable older adults.
Preoperative colonic examination is recommended to evaluate for synchronous carcinoma or neoplastic polyps, and may alter treatment in up to one-third of patients [15]. For vulnerable older adults who have a higher burden of comorbid disease and lower physiologic reserve the consequences of missed colonic pathology can be serious. If the additional tumor or polyp is discovered intra-operatively by the surgeon, the result may be a longer procedure and a more extensive resection, leading to increased risk of perioperative morbidity and mortality. If the colonic neoplastic lesion is not discovered until later, the patient may be exposed to the risk associated with a second operation, and there may also be a delay or interruption in adjuvant chemotherapy. In the current study adherence to staging recommendations regarding endoscopic evaluation of the colon was satisfactory - a total of 87% of patients had either SCOPE alone or SCOPE plus SCAN in the 6 months before or after surgical resection. The group that was not staged with SCOPE had increased odds of 3-year mortality, underlining the importance of this staging modality in older adults with colon cancer.
While the rationale for routine total colonic examination in patients newly diagnosed with colon cancer is well established, the utility of preoperative abdominopelvic cross-sectional imaging has been more contentious [16-18]. The ACOVE-3 guidelines make the recommendation based on evidence that preoperative imaging aids the planning of the surgical procedure and other treatments. Prior research has shown that preoperative imaging of the abdomen and pelvis alter treatment plan in 16-19% of patients, either by substantially changing the surgical procedure when respectable extensive or distant disease is found, or by demonstrating unresectable metastatic disease for which the best treatment may be chemotherapy or other palliative measures, including referral to hospice care [16,19]. Given the potential significance of preoperative imaging findings in patients with colon cancer who are considering surgical therapy, the finding that only 50% of the patients in the current study had a preoperative CT or MRI is concerning.
Early identification of metastatic disease allows the avoidance of a major surgery, earlier initiation of chemotherapy, and timely, more meaningful discussions of goals of care as well as earlier hospice referral. Interestingly, while adherence to the recommended guidelines for both colonic examination and abdominopelvic imaging reduced the risk of 3-year mortality, patients who only received imaging were actually found to have increased odds of death (OR=1.22, 95% CI=1.08-1.38). Patients who were staged with SCAN alone may have had contraindications to SCOPE related to comorbid illness, thus comprising a higher-risk subgroup that would be expected to have poorer perioperative outcomes and long-term survival.
Beyond examining the frequency of adherence to preoperative staging guidelines for colon cancer, we also assessed the association of patient- and disease-related factors with guideline adherence. Predictors of adherence included Black and Asian/Pacific Islander/ Hispanic race/ethnicity, hospitalization within the previous year, higher tumor stage, moderate or poorly differentiated tumor grade, and non-emergent hospitalization.
Some of these factors such as non-White race and advanced tumor stage and grade define populations at higher risk for worse cancer related outcomes, and it is possible that treating physicians had a higher index of suspicion for advanced or metastatic disease and therefore ordered staging studies. Patients hospitalized within the prior year may have had increased utilization of SCAN and SCOPE in the work-up of other medical problems, or it could be that their engagement within the health care system related in earlier colon cancer diagnosis. The finding that patients who presented emergently with colon cancer had decreased preoperative imaging is not surprising, as both SCAN and SCOPE may not be appropriate or possible to perform in the emergent setting. We also found a discrepancy in adherence to staging recommendations by SEER region; such geographic variations in the delivery of cancer-related and other health care is well-described [20,21]. The geographic discrepancy hints at the integral role of the systems-wide process, and could allow for specific models for the structuring of the diagnostic work-up of colon cancer patients to both increase guideline adherence while reducing therapeutic delays [22].
Our study has several limitations. We were unable to examine the impact of patient preferences and physician recommendations on decision-making related to preoperative staging. For patients who did not undergo either SCAN or SCOPE, we were not able to determine why these diagnostic studies were omitted. The study period spanned over a decade, and it was only towards the end of this time period that consistent recommendations regarding preoperative staging of colon cancer were published by national and international organizations [23-28]. Our survival analysis has the potential for significant confounding factors, including the presence of non-cancer comorbidities and other clinical factors which impact survival as well as the ability to get preoperative staging.
However, despite these limitations, this study is the first to examine patterns of colon cancer staging using population-level data and important implications. The study demonstrated the existence of a significant gap in adherence to staging guidelines in older adults with colon cancer. Given the association between non-adherence and increased mortality, it is imperative that interventions are devised to improve adherence to colon cancer staging guidelines in vulnerable older adults.
Conclusion
Adherence with published quality indicators improved outcome and survival for vulnerable elders with potentially resectable colorectal cancer.
Funding Source
University of Wisconsin Carbone Cancer Center Support Grant from the National Cancer Institute - National Institutes of Health, grant number P30CA014520-34.
References
- 65+ in the United States: 2005, 2005.
- Faivre-Finn C, Bouvier-Benhamiche AM, Phelip JM, Manfredi S, Dancourt V, Faivre J. Colon cancer in France: evidence for improvement in management and survival. Gut. 2002; 51: 60-64.
- Kunitake H, Zingmond DS, Ryoo J, Ko CY. Caring for octogenarian and nonagenarian patients with colorectal cancer: what should our standards and expectations be? Dis Colon Rectum. 2010; 53: 735-743.
- Wenger NS, Roth CP, Shekelle P; ACOVE Investigators. Introduction to the assessing care of vulnerable elders-3 quality indicator measurement set. J Am Geriatr Soc. 2007; 55: S247-252.
- Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001; 49: 1691-1699.
- Donabedian A. The quality of care. How can it be assessed? JAMA. 1988; 260: 1743-1748.
- McGory ML. Quality indicators for the care of colorectal cancer in vulnerable elders. J Am Geriatr Soc. 2007; 55 Suppl 2: S277-284.
- Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002; 40: IV-3-18.
- Cooper GS, Virnig B, Klabunde CN, Schussler N, Freeman J, Warren JL. Use of SEER-Medicare data for measuring cancer surgery. Med Care 2002; 40: IV-43-IV-8.
- Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993; 31: 732-748.
- Lo NS, Sarr MG. Mucinous cystadenocarcinoma of the appendix. The controversy persists: a review. Hepatogastroenterology. 2003; 50: 432-437.
- Mitchell DG, Parker L, Sunshine JH, Levin DC. Body MR imaging and CT volume: variations and trends based on an analysis of medicare and fee-for-service health insurance databases. AJR Am J Roentgenol. 2002; 179: 27-31.
- Gross CP, Andersen MS, Krumholz HM, McAvay GJ, Proctor D, Tinetti ME. Relation between Medicare screening reimbursement and stage at diagnosis for older patients with colon cancer. JAMA. 2006; 296: 2815-2822.
- Ash AS, Ellis RP, Pope GC, Ayanian JZ, Bates DW, Burstin H, et al. Using diagnoses to describe populations and predict costs. Health Care Financ Rev. 2000; 21: 7-28.
- Isler JT, Brown PC, Lewis FG, Billingham RP. The role of preoperative colonoscopy in colorectal cancer. Dis Colon Rectum. 1987; 30: 435-439.
- Barton JB, Langdale LA, Cummins JS, Stelzner M, Lynge DC, Mock CN, et al. The utility of routine preoperative computed tomography scanning in the management of veterans with colon cancer. Am J Surg. 2002; 183: 499-503.
- McAndrew MR, Saba AK. Efficacy of routine preoperative computed tomography scans in colon cancer. Am Surg. 1999; 65: 205-208.
- Mayes GB, Zornoza J. Computed tomography of colon carcinoma. AJR Am J Roentgenol. 1980; 135: 43-46.
- Mauchley DC, Lynge DC, Langdale LA, Stelzner MG, Mock CN, Billingsley KG. Clinical utility and cost-effectiveness of routine preoperative computed tomography scanning in patients with colon cancer. Am J Surg 2005; 189: 512-517.
- Fischer ES, Wennberg DE, Stukel TA, Gottlieb DJ, Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 1: the content, quality, and accessibility of care. Ann Intern Med 2003; 138: 273-287.
- Fischer ES, Wennberg DE, Stukel TA, Gottlieb DJ. Lucas FL, Pinder EL. The implications of regional variations in Medicare spending. Part 2: health outcomes and satisfaction with care. Ann Intern Med 2003; 138: 288-298.
- Klemann VM, Wolters FL, Konsten JL. Benefits of a well-structured diagnostic process in colon cancer. Dig Surg. 2011; 28: 15-21.
- NCCN Clinical Practice Guidelines in Oncology FColon Cancer. 2014.
- Otchy D, Hyman NH, Simmang C, Anthony T, Buie WD, Cataldo P, et al. Practice parameters for colon cancer. Dis Colon Rectum. 2004; 47: 1269-1284.
- Tveit KM, Kataja VV; ESMO Guidelines Taskforce. ESMO minimum clinical recommendations for diagnosis, treatment and follow-up of rectal cancer. Ann Oncol. 2005; 6: i20-i21.
- Van Cutsem EJ, Kataja VV; ESMO Guidelines Taskforce. ESMO minimum clinical recommendations for diagnosis, adjuvant treatment and follow-up of colon cancer. Ann Oncol. 2005;16: i16-i17.
- Desch CE, Benson AB 3rd, Smith TJ, Flynn PJ, Krause C, Loprinzi CL, et al. Recommended colorectal cancer surveillance guidelines by the American Society of Clinical Oncology. J Clin Oncol. 1999; 17: 1312.
- Benson AB 3rd, Desch CE, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, et al. 2000 update of American Society of Clinical Oncology colorectal cancer surveillance guidelines. J Clin Oncol. 2000; 18: 3586-3588.